This week, millions of Americans became eligible to receive a booster shot of the Pfizer-BioNTech COVID-19 vaccine.
In a press briefing on Friday, President Joe Biden revealed that at least 60 million people can start receiving third doses – but told those outside the eligible groups to ‘wait their turn.’
But there has been some confusion about whom Biden was talking about, especially after Centers for Disease Control and Prevention Director (CDC) Dr Rochelle Walensky overruled her own agency’s advisory panel to recommend boosters for a broader group.
As this new phase of the vaccination rollout begins, DailyMail.com breaks down who is eligible to receive boosters, where they can get them and why CDC advisers were at odds with the U.S. Food and Drug Administration (FDA).

On Friday, President Joe Biden announced at a press briefing (above) that 60 million Americans have become eligible to receive booster shots of the COVID-19 vaccine
WHAT ARE COVID-19 VACCINE BOOSTERS?
A booster shot is given at least six months after people have been fully vaccinated against COVID-19.
It is meant to prolong immunity and give a ‘boost’ to the immune system to create higher levels of antibodies against the virus.
WHICH COVID-19 VACCINE BOOSTER CAN I GET?
Right now, only people who received two doses of the Pfizer-BioNTech vaccine, and were given their final shot at least six months ago, can get booster shots.
This is because the FDA has only reviewed data from Pfizer so far.
Pfizer’s booster shot is exactly the same – ingredients-wise and dosage (30 micrograms) – as the first two doses.
WHO SHOULD GET BOOSTER SHOTS?
Last month, boosters were authorized for Americans with compromised immune systems. This week, that authorization was expanded to specific at-risk groups.
The CDC’s Advisory Committee on Immunization Practices (ACIP) recommended on Thursday that people in any of these groups should get a third dose:
People aged 65 and olderLong-term care facility residentsPeople aged 50 to 64 at high risk of severe COVID-19 due to underlying medical conditions 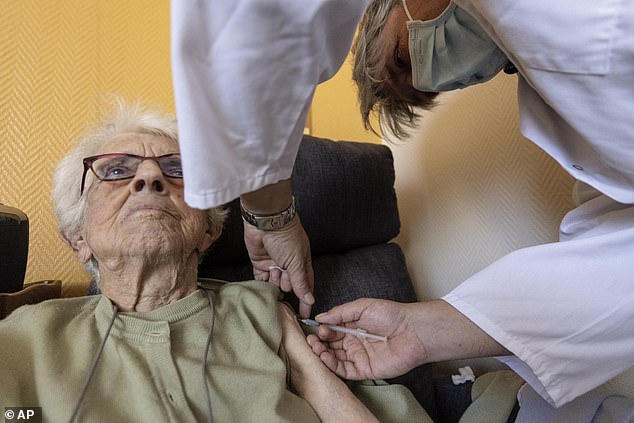
DailyMail.com has provided a breakdown of who is eligible for boosters, when they can get them and why a CDC panel initially rejected boosters for specific groups. Pictured: An elderly woman gets a COVID-19 Pfizer booster, September 24
WHO CAN CONSIDER GETTING BOOSTERS?
Two more groups were made eligible by the CDC, but stopped short of offering a full recommendation, instead saying they can consider boosters:
People aged 18 to 64 with underlying health conditions based on individual benefits and risks People aged 18 to 64 at high-risk due to their job, such as healthcare workers, teachers and grocery store employees -People aged 18 to 64 who are in homeless shelters or in prisons
BUT DIDN’T THE CDC’S ADIVSORY PANEL RECOMMEND AGAINST BOOSTERS FOR PEOPLE AT HIGH RISK DUE TO THIEIR JOBS?
ACIP voted 8-7 against recommending use for those are at risk due to an ‘occupational or institutional setting’ after the FDA authorized third doses for this group.
During its meeting on Thursday, some members said the FDA had made this authorization based on limited data and there was not enough evidence to prove that people in these groups needed third doses.
Others were concerned that approving boosters based on employment would lead to excessively broad use.
But, in a surprise move, Walensky overturned the decision on Thursday night, saying that giving boosters to healthcare workers and others would ‘best serve the nation’s public health needs.’
‘In a pandemic, even with uncertainty, we must take actions that we anticipate will do the greatest good,’ she said in a statement.
Biden agreed with this move because it is in line with his own administration’s plan to eventually offer boosters all Americans.
WHERE CAN I GET A BOOSTER SHOT?
During a press briefing on Friday, Biden said that booster shots will be available in 80,000 locations across the U.S.
This includes more than 40,000 pharmacies as well as health departments, hospitals and clinics.
You will likely have to show your vaccine card and proving that you fall under one of the qualifying groups will be on an honor system rather than providing proof.
Under the FDA’s emergency use authorization, boosters are free.
HAVE BOOSTERS SHOTS BEEN APPROVED BECAUSE PROTECTION FROM THE VACCINES IS WANING?
Recently, some studies have suggested that certain groups of people – and certain vaccines – may have waning immunity over time.

Boosters were approved after several reports, including a CDC study, showed the Moderna jab was 96.3% effective against symptomatic illness while the Pfizer shot was 88.9% effective – and the Moderna jab was also more effective after one dose
Some people have weakened immune systems, either due to medical conditions or to age, that have left them unable to mount a full immune response to the first two doses.
Additionally, recent studies have found that vaccine protection does decrease after more than four months.
This week, a study led by the CDC compared 5,000 healthcare workers in 25 states who received either the Pfizer vaccine or the Moderna vaccine
After two doses, the Moderna jab was 96.3 percent effective against symptomatic illness while the Pfizer shot was 88.9 percent effective.
What’s more, a CDC report issued last week, found the Moderna vaccine was 93 percent effective against hospitalization compared to 88 percent for the Pfizer vaccine and 71 percent for the Johnson & Johnson jab.
The report also found that antibody levels in blood samples were highest among Moderna recipients in a separate group of healthy volunteers compared to levels for Pfizer and J&J recipients.
However, health officials insist that vaccines are still highly effective against the most severe outcomes from COVID-19, including hospitalization and death.
They also say they want to focus rollout on getting the unvaccinated, who are the highest risk of serious outcomes, their shots.
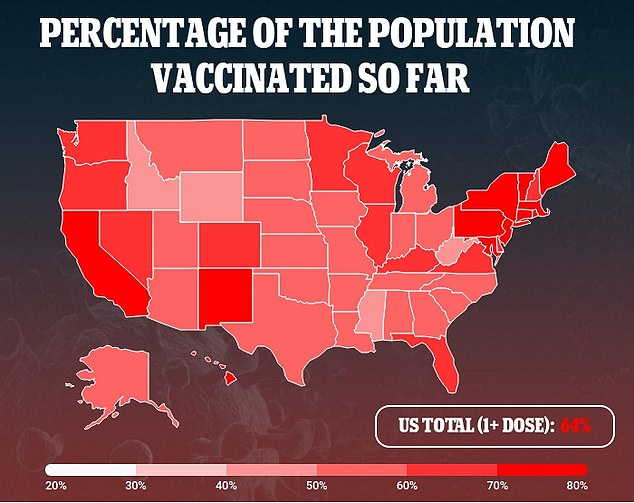
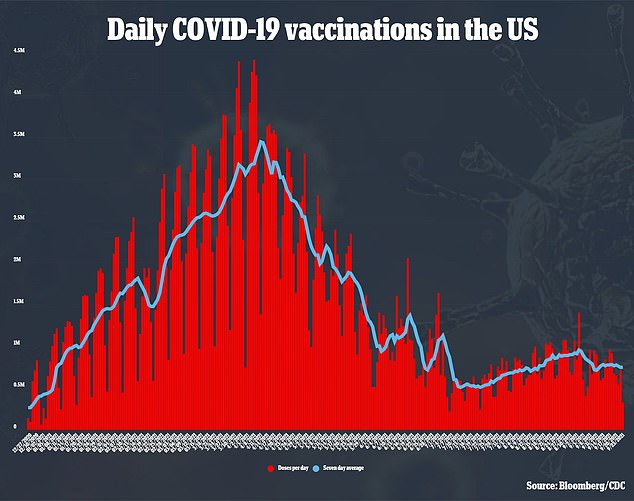
WHAT IF I RECEIVED THE MODERNA OR JOHNSON & JOHNSON VACCINES?
Moderna has submitted an application to the FDA asking that its booster shot be authorized while Johnson & Johnson is expected to do so in the coming weeks.
Because of this, recipients of either of these two vaccines are not eligible to receive boosters yet – but both are expected to be in the future.
Biden said on Thursday that scientists are still examining data for boosters shots from the two companies.
‘Our doctors and scientists are working day and night to analyze the data from those two organizations on whether and when you need a booster shot, and we’ll provide updates for you as the process moves ahead,’ he said.
CAN I MIX AND MATCH?
Currently, federal health officials do not recommend getting a booster shot made by a different vaccine manufacturer than that of your initial doses.
This means that Moderna and Johnson & Johnson recipients are not recommended to get a booster dose from Pfizer and vice-versa.
Source link : https://www.dailymail.co.uk/health/article-10026225/DailyMail-com-breaks-need-know-COVID-19-vaccine-boosters.html











