The U.S. Food and Drug Administration (FDA) is aiming to give full approval to Pfizer-BioNTech’s COVID-19 vaccine by early next month.
People involved in the effort told The New York Times that the federal health agency has accelerated the timeline for approval from January 2022 to an unofficial deadline of Labor Day on September 6.
The FDA gave emergency use authorization to the Pfizer coronavirus vaccine in mid-December 2020, making it the first to be distributed in the U.S.
Full approval by the FDA could push more Americans to get the COVID-19 vaccine as it might reduce their fears about the safety of the shot.
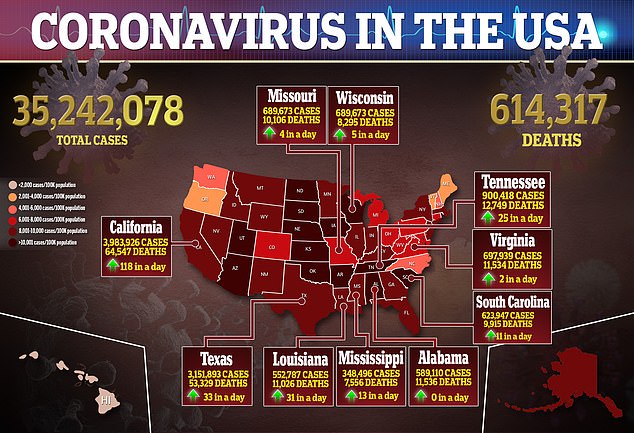
It comes as the country enters its fourth wave of the pandemic with average daily cases surpassing 90,000, which is the highest figure recorded since February.
The FDA is hoping to grant full approval to Pfizer-BioNTech’s coronavirus vaccine by early September with an unofficial deadline of Labor Day. Pictured: A vial of the Pfizer-BioNTech COVID-19 vaccine in Potsdam, Germany, January 2021
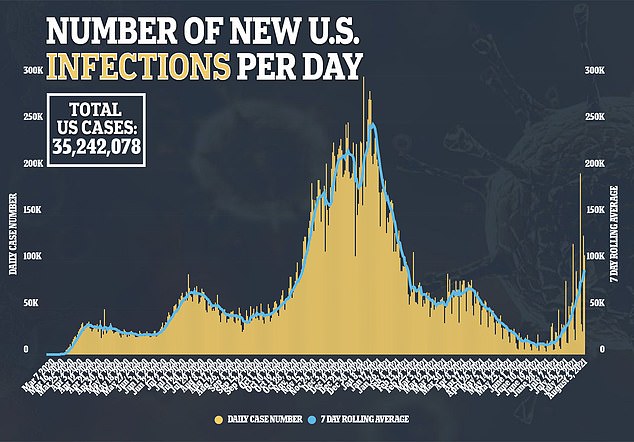
It comes as average daily cases surpass 90,000, which is the highest figure seen since February and a 283% jump in the last three weeks
Because Pfizer’s vaccine is currently approved for use on an emergency basis, it is still considered somewhat experimental despite data showing it safe and effective.
Additionally, emergency use authorization requires less clinical trial data, with the FDA only requiring two months of follow-up before approving the shot for those 16 and older last year compared to six months for full approval.
In a statement to DailyMail.com, an FDA spokeswoman said the agency could not comment on specific timing, but has been moving as quickly as possible to review Pfizer’s application.
‘Acknowledging the urgency related to the current state of the pandemic, we have taken an all-hands-on-deck approach, including identifying additional resources such as personnel and technological resources from across the agency and opportunities to reprioritize other activities, in order to complete our review to help combat this pandemic surge,’ the statement read.
The designation is also intended to be temporary.
If and when the shot is fully approved, companies and schools may feel more comfortable requiring employees and students to get it.
A recent report from the Kaiser Family Foundation found three in ten unvaccinated adults said they would be more likely to get vaccinated if one of the vaccines were fully approved.
However, the researchers warned that most unvaccinated respondents did not understand the FDA approval process and may just be looking for a reason to not get vaccinated.
‘If vaccines are fully authorized, that would take that excuse [for not getting vaccinated] off the table,’ Dr William Schaffner, a professor of preventive medicine and infectious diseases at the Vanderbilt University Medical Center, told DailyMail.com in an interview last week.
‘If fully licensed, I think that movement of [vaccine] mandates would accelerate and generate lots of vaccinations.’
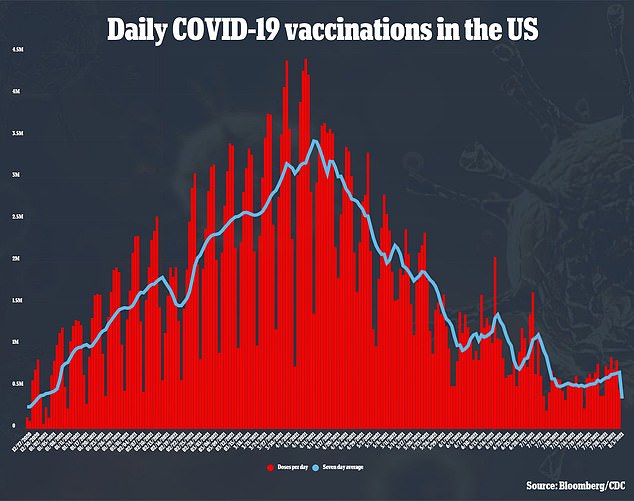
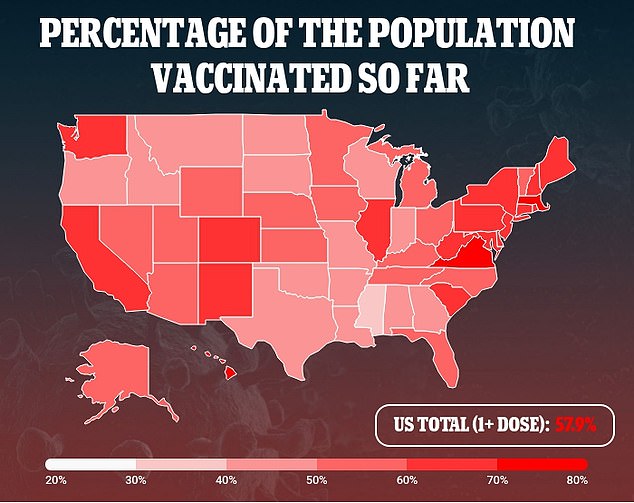
The decision would also allow the vaccine makers to market their shots directly to the general public.
President Joe Biden said last week he expected the FDA to make the decision by early fall, but didn’t provide any additional details.
The spread of the highly contagious Indian ‘Delta’ Covid variant has fueled a new surge in infections.
Cases have risen in nearly 90 percent of U.S. jurisdictions, according to the Centers for Disease Control and Prevention (CDC).
What’s more, average daily infections have risen 283 percent in the last three weeks from 23,613 to 90,576 – Johns Hopkins data show – and are currently at their highest levels since February 2021.
Deaths, which are a lagging indicator have also begun rising after holding flat for about a month, by 53.3 percent in the last three weeks from an average of 260 virus-related fatalities ore day to an average of 399 per day.
Source link : https://www.dailymail.co.uk/health/article-9858495/FDA-aiming-final-approval-Pfizer-vaccine-early-month-NY-Times.html











