The U.S. Food and Drug Administration (FDA) plans on giving full approval to the Pfizer-BioNTech COVID-19 vaccine by Monday, according to The New York Times.
The two-dose vaccine was the first to receive emergency use authorization in December 2020.
Sources told told The Times that the FDA had previously expected to approve the vaccine before Labor Day, but decided to expedite the timeline.
Regulators are currently working through paperwork and final negotiations with the company before the Pfizer vaccine becomes the first to receive full authorization.
Full approval by the FDA could push more Americans to get the COVID-19 vaccine as it might reduce their fears about the safety of the shot.
The FDA plans to give the Pfizer-BioNTech COVID-19 vaccine full approval by Monday. Pictured: A Pfizer COVID-19 vaccine vial is pictured at a vaccine mobile clinic in Los Angeles, July 2021

The FDA plans to give the Pfizer-BioNTech COVID-19 vaccine full approval by Monday, according to anonymous sources who spoke to The New York Times (file image)
The Pfizer vaccine is the most common in the U.S., having been administered over 200 million times in the last nine months, according to data from the Centers for Disease Control and Prevention (CDC).
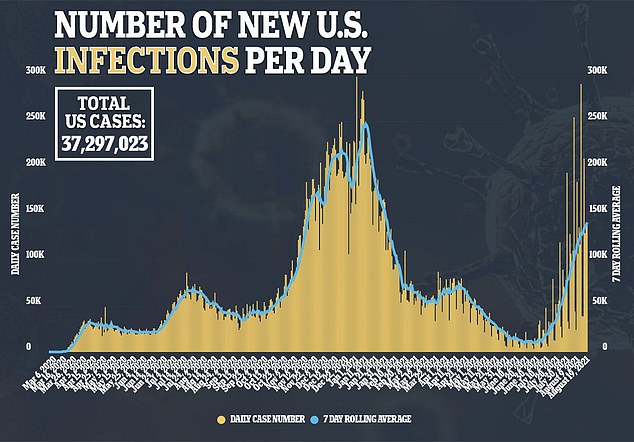
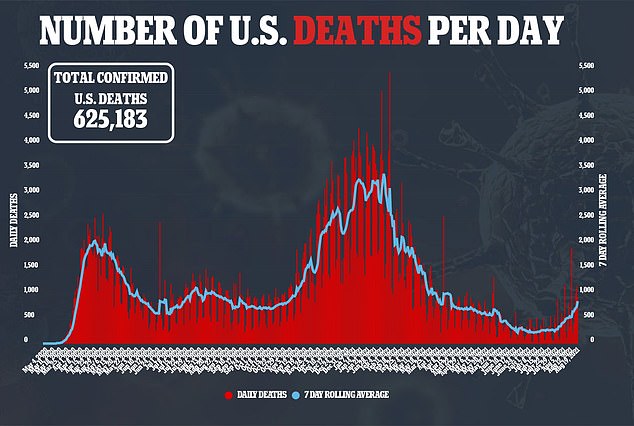
News of full approval comes amid the country’s fourth wave of the pandemic.
Daily COVID-19 cases have increased by 600 percent from 20,000 cases per day on July 1 to over 140,000 cases per day in mid-August.
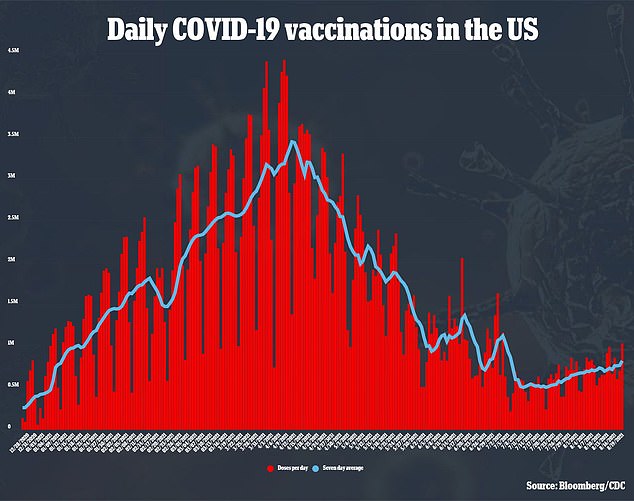
Demand for the vaccines has grown in recent weeks as well, as the surge in cases has pushed more Americans into getting the shots.
As of now, around 750,000 Americans are receiving a vaccine dose every day, and more than one million doses were distributed in a 24 hour span this week – the first time that has happened since July 3.
The federal government also recently announced plans to roll out third doses of the Pfizer and Moderna vaccines, starting on September 20.
Pfizer’s vaccine is currently approved for use on an emergency basis, it is still considered somewhat experimental despite data showing it is safe and effective.
Emergency use authorization requires less clinical trial data, with the FDA only requiring two months of follow-up before approving the shot for those 16 and older last year compared to six months for full approval.
The designation is also intended to be temporary.
When the shot is fully approved, companies and schools may feel more comfortable requiring employees and students to get it.
A recent report from the Kaiser Family Foundation found three in ten unvaccinated adults said they would be more likely to get vaccinated if one of the vaccines were fully approved.
However, the researchers warned that most unvaccinated respondents did not understand the FDA approval process and may just be looking for a reason to not get vaccinated.
‘If vaccines are fully authorized, that would take that excuse [for not getting vaccinated] off the table,’ Dr William Schaffner, a professor of preventive medicine and infectious diseases at the Vanderbilt University Medical Center, told DailyMail.com in an interview last month.
‘If fully licensed, I think that movement of [vaccine] mandates would accelerate and generate lots of vaccinations.’
The decision would also allow the vaccine makers to market their shots directly to the general public.
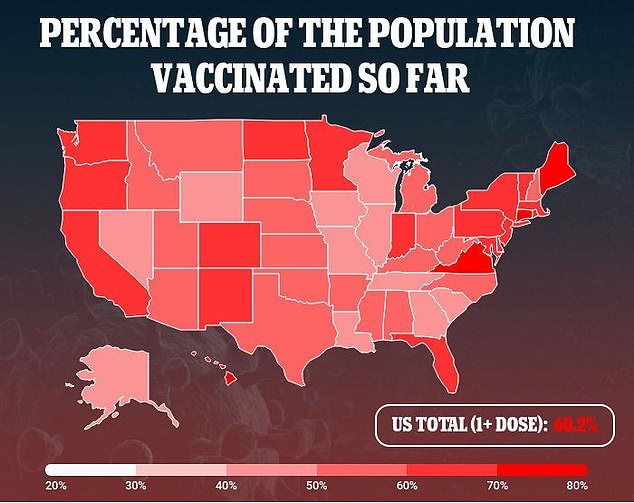
Currently, more than 60 percent of people have received at least one shot of a COVID-19 vaccine, and 51 percent are fully vaccinated.
Anyone over the age of 12 is eligible for the Pfizer vaccine.
Moderna, which produced a similar mRNA vaccine to Pfizer that also received emergency use authorization in December, has also submitted an application for full FDA approval.
This is a breaking news story and will be updated.
Source link : https://www.dailymail.co.uk/health/article-9913377/FDA-planning-grant-approval-Pfizers-COVID-19-vaccine-Monday.html











