Merck & Co announced on Monday that it has applied for emergency use authorization with the U.S. Food and Drug Administration (FDA) for its experimental pill to treat mild-to-moderate COVID-19 patients.
The drug, called molnupiravir, stops the virus from making copies of itself, which prevents it from spreading throughout the body.
Recent trial data have shown that it can halve the chances of death or being hospitalized for those most at risk of contracting a severe case of Covid.
If authorized, Merck’s drug could be the first oral antiviral medication for COVID-19 – and could allow patients to take the drug at home instead of requiring them to go to a hospital for treatment.

Merck & Co asked the FDA for emergency use authorization of its pill – the very first – to treat mild-to-moderate COVID-19 patients. Pictured: A Merck sign stands in front of the company’s building in Summit, New Jersey
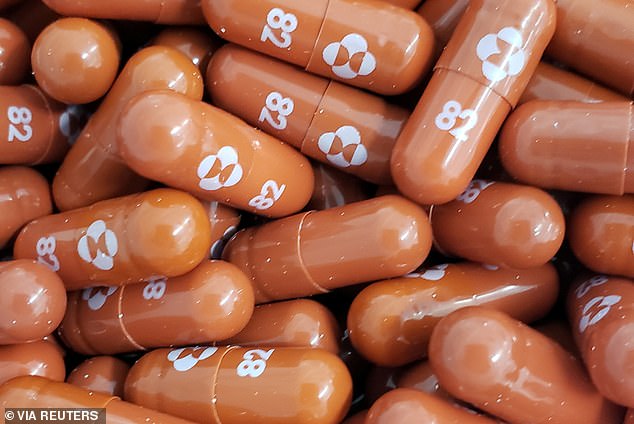
The drug, molnupiravir (pictured), stops viruses like the coronavirus from making copies of itself and spreading throughout the body
Molnupiravir is an antiviral drug that was developed at Emory University, in Atlanta, by its drug innovation company, Drug Innovation Ventures at Emory (DRIVE), which was licensed by Ridgeback Biotherapeutics LP, who partnered with Merck.
It was originally meant to treat influenza and prevents the virus from making copies of itself by creating errors during viral RNA replication.
Animal studies conducted last year found molnupiravir could completely suppress viral transmission and prevent and reduce severe lung damage.
The new study tracked 775 adults with mild-to-moderate COVID-19 who were considered higher risk for severe illness due to health problems such as obesity, diabetes or heart disease.
Among patients taking molnupiravir, 7.3 percent were either hospitalized or died at the end of 30 days, compared with 14.1 percent of those getting the dummy pill.
There were no deaths in the drug group after that time period compared with eight deaths in the placebo group, according to Merck.
The results were released by the company and have not been peer-reviewed, but Merck says it plans to present them at a future medical meeting.
An independent group of medical experts monitoring the trial recommended stopping it early because the interim results were so strong.
‘It exceeded what I thought the drug might be able to do in this clinical trial,’ Dr Dean Li, vice president of Merck research, told the Associated Press.
‘When you see a 50 percent reduction in hospitalization or death, that’s a substantial clinical impact.’
Side effects were reported by both groups in the Merck trial, but they were slightly more common among the group that received a dummy pill. The company did not specify the problems.
The interim efficacy data on the drug had heavily impacted the shares of COVID-19 vaccine makers when it was released last week.
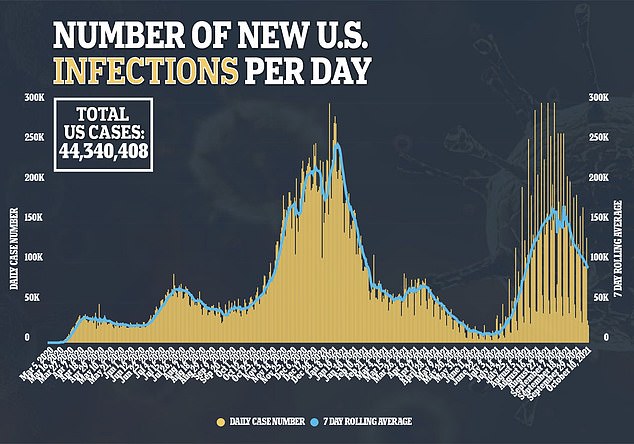
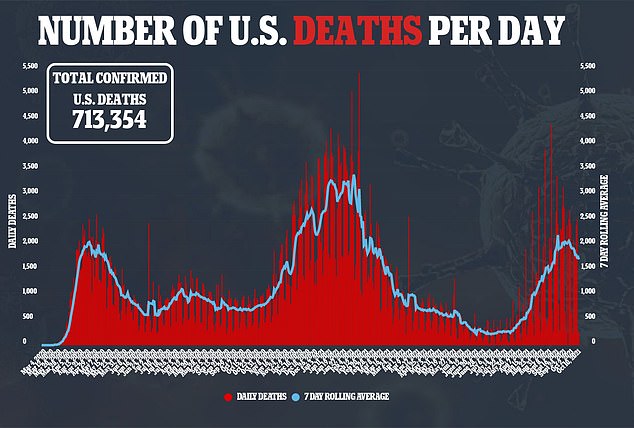
Existing drugs from Gilead Sciences Inc’s infused antiviral remdesivir and generic steroid dexamethasone are generally given only once a patient is hospitalized.
Monoclonal antibody drugs from Regeneron Pharmaceuticals Inc and Eli Lilly have so far seen only limited use due to the difficulty in administering them.
In India, however, two drugmakers had last week sought to end late-stage trials of their generic versions of molnupiravir to treat moderate COVID-19, according to study documents.
A source with the Drug Controller General of India had said the pill has not shown ‘significant efficacy’ against moderate cases, but was successful against mild cases.
Merck said its trials are based on fda definitions, which for moderate COVID-19 describe blood oxygen levels as no lower than 93 percent whereas the trials in India define moderate as blood oxygen levels between 90 percent and 93 percent.
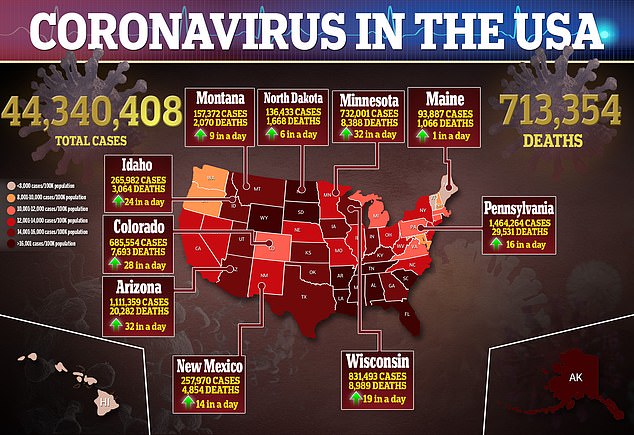
Source link : https://www.dailymail.co.uk/health/article-10079873/Merck-seeks-U-S-FDA-authorization-COVID-19-tablet.html











