Moderna and Johnson & Johnson are pushing to get COVID-19 vaccine boosters approved ahead of a key U.S. Food and Drug Administration (FDA) advisory committee meeting later this week.
The two firms say that data in briefing documents, published on Tuesday by the FDA, show extra doses are needed.
Moderna said the data support the use of boosters for people aged 18 and older six months after receiving the second shot, citing the waning effectiveness of the shots at preventing infection, and the increased antibody levels provided by third shots.
J&J is also vying for its booster shot to be given to adults six months after they received the first dose, and said its clinical trial data showed boosters should also be given to adults.
The New Brunswick, New Jersey-based company also says those who are especially at high risk should receive the second shot as early as two months after the first shot.
On Thursday, a two-day advisory advisory meeting will commence to discuss whether the companies’ boosters are needed.
Moderna is hoping its COVID-19 vaccine booster shot will receive authorization for people over the age of 65 or with serious comorbidities – the same as the Pfizer-BioNTech vaccine received (file image)
Johnson & Johnson is hoping a second shot of its COVID-19 vaccine will be authorized for use six months after receival of the first shot, or only two months for people with serious vulnerabilities to Covid (file iamge)
Currently, the Pfizer-BioNTech vaccine is the only shot with a booster dose authorized for those aged 65 or older or who at high risk of severe disease due to underlying condition or their jobs.
A booster dose can also be given to immunocompromised Americans as can a third shot of Moderna’s vaccine.
Official data from the Centers for Disease Control and Prevention (CDC) show that 7.8 million doses of COVID-19 vaccine boosters have been distributed as of Tuesday.
A vast majority, 6.2 million, are of the Pfizer vaccine, though just under 1.5 million doses of the Moderna booster have been distributed as well.
Some of these shots are unauthorized, being given out by primary care physicians and other doctors at regular check ups.
Moderna, based in Cambridge, Massachusetts, is now pushing to get its booster as widely distributed as Pfizer’s, and have eligibility expanded.
Moderna’s booster shot will be discussed by the FDA advisory panel on Thursday.
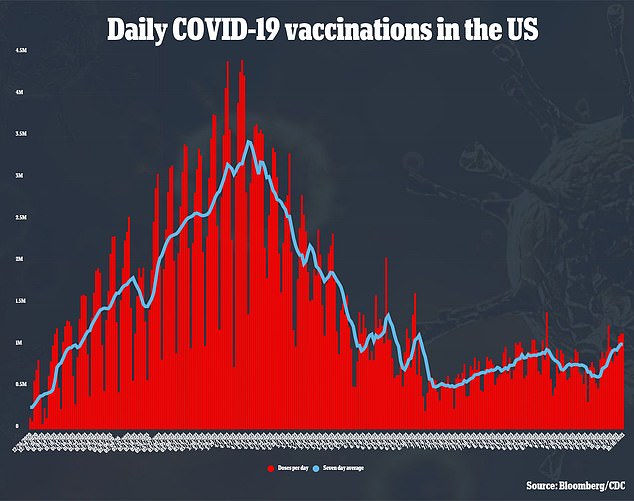
Booster shots for the Moderna vaccine were expected to roll out to all Americans 18 or older on September 20.
The White House announced in late August that the Pfizer and Moderna vaccines would both have boosters available in late September, pending approval from regulators like the FDA.
Despite the waning efficacy of the vaccines, the shots are still deemed to be effective at preventing hospitalization or death.
Because of this, regulators did not approve the shots by the September 20 deadline, and even when the Pfizer booster was eventually authorized it did not receive the full clearance for all Americans that the White House was aiming for.
Meanwhile, J&J makes the only one-shot vaccine available in the U.S., and is hoping to gain authorization for second shots later this week.
The company reports some rare side-effects such as blood clotting or heart inflammation in people who received the booster, but not to the extent that it believes the shot is unsafe.
Authorization for the second shots of the J&J vaccine will be discussed by the advisory panel on Friday.
Initial regimens of the J&J and Moderna COVID-19 vaccines are available to all adults in the U.S.
The Pfizer vaccine – unlike the other two – is also available to minors between the ages of 12 to 17.
More than 150 million doses of the two-shot Moderna vaccine have been used in the U.S since the shot received authorization in December – trailing the Pfizer shot by 80 million – and 15 million doses of Johnson & Johnson have been administered.
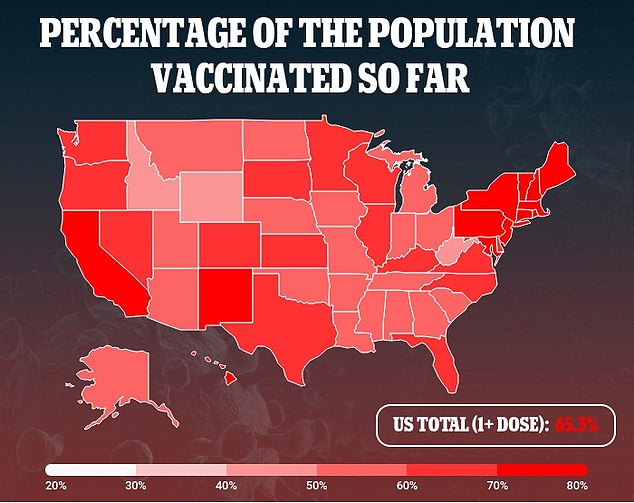
Nationwide, 65 percent of Americans have received at least one dose of a Covid vaccine, and 56 percent are fully vaccinated.
Booster shots are not yet accounted for in the definition of ‘fully vaccinated’ so anyone who receives two shots of Pfizer or Moderna or one shot of J&J is considered fully vaccinated.
Source link : https://www.dailymail.co.uk/health/article-10084103/Moderna-pushes-COVID-19-vaccine-boosters-older-adults-high-risk-individuals.html











