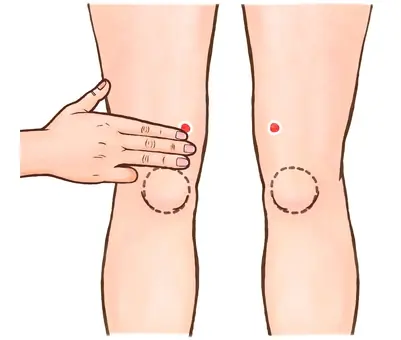
Try Gently Pressing These 2 Points Behind Your Knees — Some People Say It Helps the Body Relax
Two Pressure Points Behind Your Knees You Should Know

Two Pressure Points Behind Your Knees You Should Know

When Should You Avoid Bananas? 2 Situations to Know

7 Incredible Benefits of Kakadu Plum for Your Health

No matter how expensive they are, you should never eat these foods after they have been left overnight. Don’t gamble with your health!

The Real Advantages of Starting Your Day with Coffee

12 Health Benefits of Starting Your Day with Banana and Avocado for 30 Days

You can grow 8 types of snake-repelling plants 👇👇👇

The Surprising Reason Kni.ves Sometimes Have a Small Hole

According to a recent study published in The British Journal of Nutrition (UK), eating about 100g of watercress per day can prevent breast cancer and many other cancers.

Do your best to be the parent your children deserve

The Ideal Battery Percentage to Charge Your Phone for Longer Lifespan

Which Is Better When Buying Garlic: White or Purple Skin?

Worried About Snakes Around Your Home? Here Are 5 Plants People Often Grow to Help Keep Them Away
A 50-Year-Old Man Died After Eating Leftovers From the Fridge: 5 Foods You Should Never Keep Overnight

Waking Up With a Headache? Here Are the Signs That May Point to Stroke Risk

If You Always Need the Bathroom After Eating, Here’s What Doctors Say


Oregano’s natural compounds may support health in several ways.

Many people eat these vegetables every day without knowing the danger.

Before buying chicken again, check these 3 warning signs.