
New research on so.lid tu.mor kil.ling method, successful in mice, awaiting clinical trials
In recent years, many preclinical studies have focused on the use of biologically derived compounds, particularly those extracted from snake and scorpion venoms, to destroy solid tumors in mouse models. These studies have demonstrated the potential to develop new anticancer therapies capable of acting through multiple mechanisms, reducing tumor size, and minimizing systemic toxicity compared to conventional chemotherapy.
One notable study utilized the peptide Smp24, isolated from the venom of the scorpion Scorpio maurus palmatus, in mice bearing solid Ehrlich carcinoma (SEC). The peptide was injected into mice with subcutaneous tumors. Results showed a significant reduction in tumor volume, near normalization of hematological and liver enzyme parameters, decreased levels of MDA and NO, and increased antioxidant enzyme activities such as GSH, SOD, and CAT. Histological analysis revealed marked apoptosis through increased expression of caspase-3 and Bax, while Ki-67 and VEGF levels were reduced, indicating inhibition of cell proliferation and angiogenesis. These findings confirm that Smp24 exerts a potent anticancer effect on solid tumor models in mice.
Another important study investigated the venom of the Egyptian snake Walterinnesia aegyptia combined with silica nanoparticles (WEV-NPs) in mice bearing breast and prostate tumors. Injection of this composite resulted in a marked reduction in tumor volume compared with controls. The venom induced the generation of reactive oxygen species (ROS), disrupted mitochondrial membrane potential, activated caspase-3, -8, and -9, and upregulated pro-apoptotic genes such as Bax and Bak, while downregulating anti-apoptotic proteins including Bcl-2, Mcl-1, and Bcl-XL. Additionally, WEV-NPs inhibited growth factor signaling pathways (IGF-1/EGF) and chemokines associated with metastasis (CXCL9/10/12/13/16), thereby suppressing tumor cell migration. The use of nanoparticles improved venom stability, selectivity, and penetration into solid tumor tissue, significantly enhancing therapeutic efficacy.
A separate study used L-amino acid oxidase (LAAO) derived from king cobra (Ophiophagus hannah) venom on mice bearing prostate cancer (PC-3) xenografts. The enzyme generated hydrogen peroxide (H₂O₂) within the tumor microenvironment, causing oxidative stress that led to apoptosis of cancer cells. Tumor size was markedly reduced in LAAO-treated mice, while healthy tissues remained largely unaffected, indicating a degree of biological selectivity.
Another approach involved using recombinant cystatin from snake venom to inhibit tumor angiogenesis. In mice with hepatocellular carcinoma (HCC) and B16F10 lung tumors, cystatin reduced microvessel density (MVD) and decreased VEGF-A165 and bFGF expression, leading to tumor hypoxia and progressive shrinkage. This mechanism is especially relevant to solid tumors, where blood vessel formation is essential for tumor growth and survival.
Some studies have also explored combining venom-derived peptides or enzymes with traditional chemotherapeutic agents such as doxorubicin or cisplatin to enhance efficacy while reducing systemic toxicity. In mice bearing H22 liver tumors, combination therapy reduced tumor mass by over 60%, compared with around 35% in chemotherapy-only groups.
Collectively, these studies demonstrate that venom-derived compounds from snakes and scorpions can effectively eliminate solid tumors in mice through multiple biological pathways, including oxidative stress induction, apoptosis activation, inhibition of proliferation and angiogenesis, and suppression of metastasis. However, these investigations remain at the preclinical stage. Issues such as systemic toxicity, optimal dosing, delivery methods, tumor selectivity, and clinical translation to humans require further in-depth research before clinical trials can be initiated.
News in the same category

Keep Your Kid.neys Healthy with These Simple, Natural Choices

Experts Say These Four Foods Could Be Part of the Reason. Smart People Have Already Given Them Up

3 Danger.ous Ways Eating Red Dates Could Ha.rm Your Health

5 Natural Drinks to Keep Your Li.ver Healthy and Detoxified

This ‘Super Fruit’ Could Be the Secret to Health, Beauty, and Youth

Can.cer Will Be Defeated If You Adopt These 11 Powerful Habits!
Frequent Drooling During Sleep? It Could Be a Sign of These Six Health Issues

5 Natural Bloo.d Cleansing Drinks to Detoxify and Boost Circulation

Doctors warn: three distinct hand signs may indicate li.ver failure. If you notice any of them, don’t delay seeing a doctor.

Is Broccoli Better Than Cauliflower? The Real Truth About Cancer Risk, Heart Health and More

What Are Eye Floaters? Here What To Do If you Start Seeing Them, According to an Eye Doctor
Everything you need to know about chronic constipation: A hidden threat to your digestive health
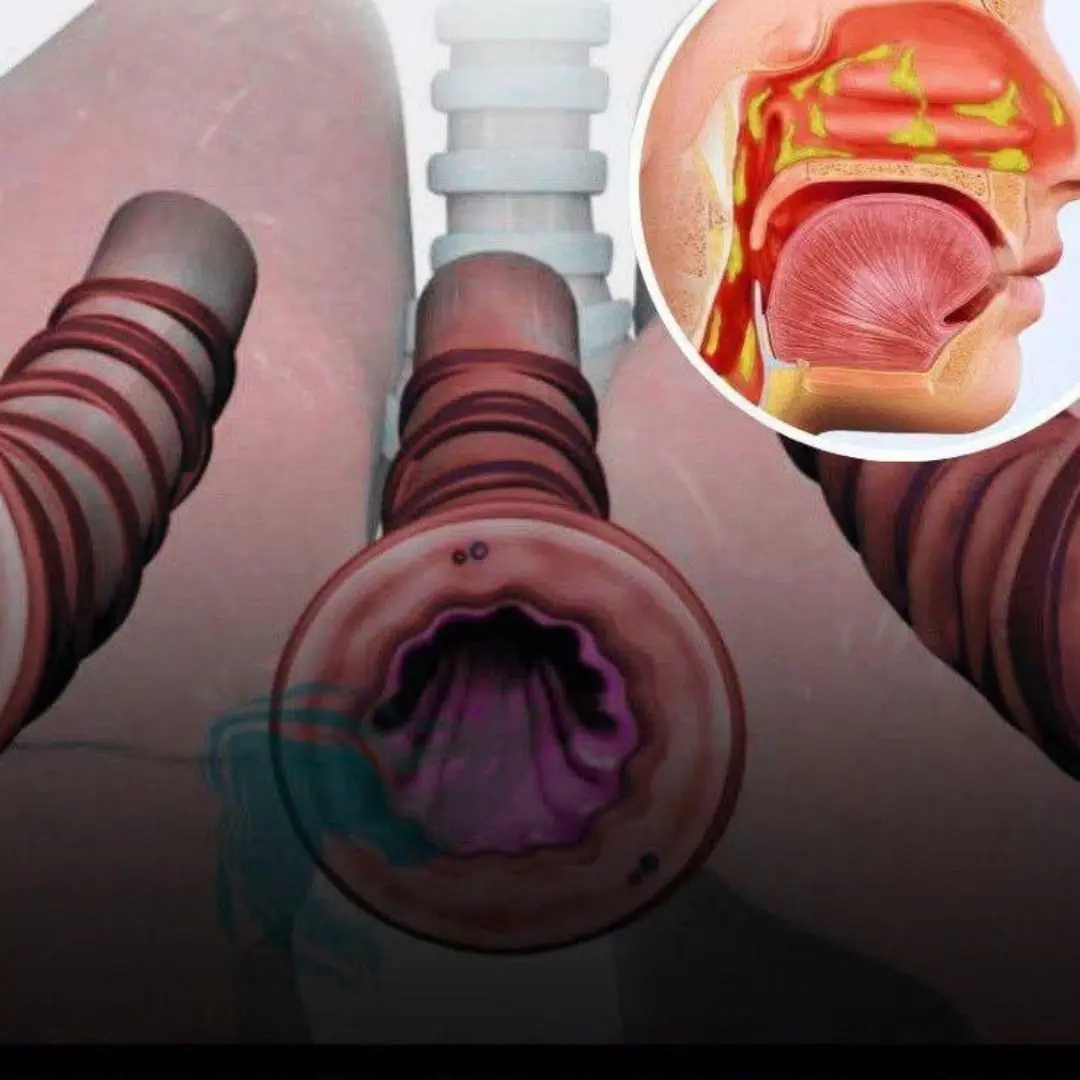
How to get rid of phlegm and mucus in your chest and throat

Doctor Shares What It Means If You Always Need To Poo Immediately After You Eat

The Reason You May Get Random Stabbing Pains in Your Chest Explained
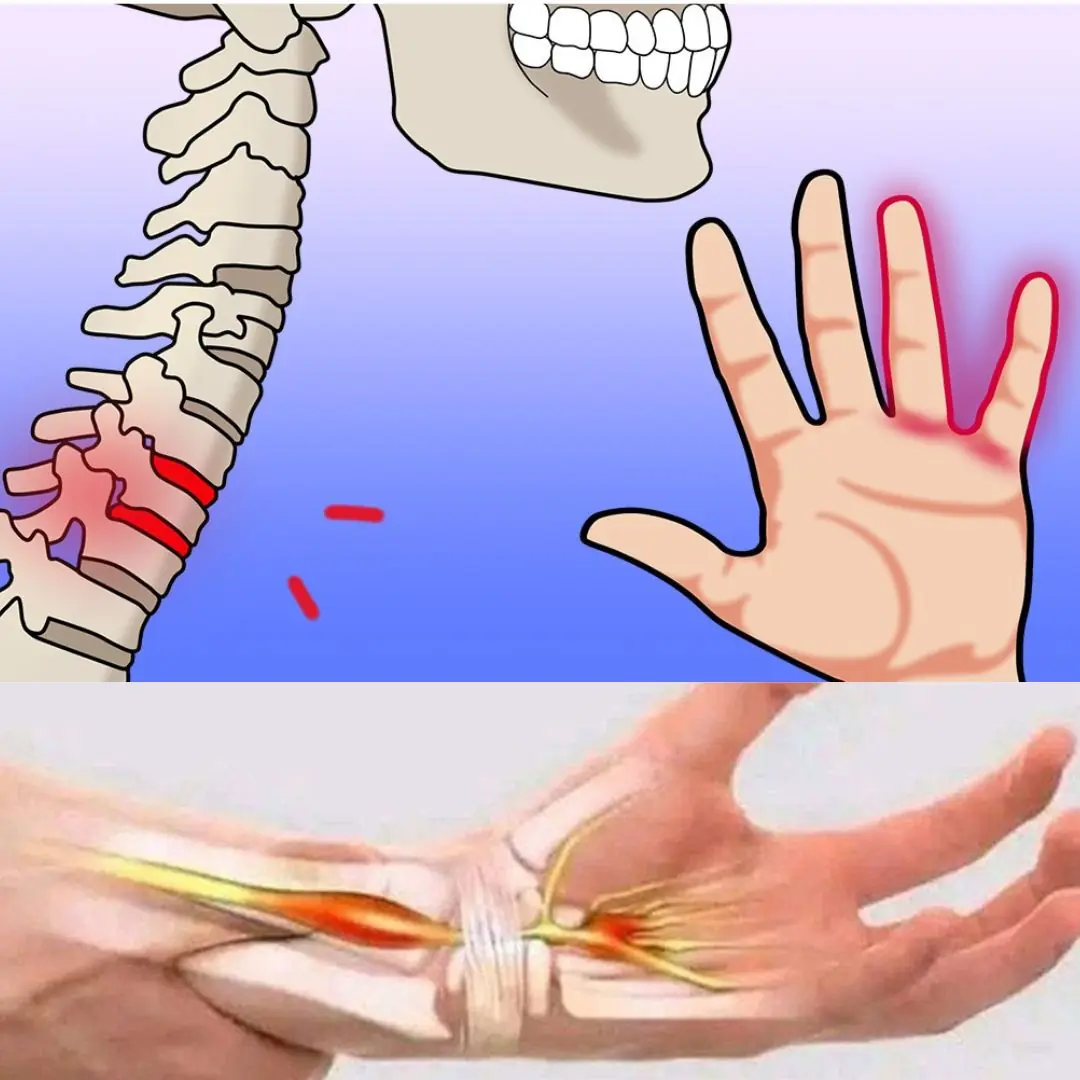
Numbness or tingling sensations in your hands

Dill Seeds: A Forgotten Treasure for Digestion, Hormones, and Restful Sleep

Eggplant is the king of vegetables but these 4 groups of people should absolutely avoid it
News Post

Snake Plant – Feng Shui Symbol or Silent Trap? 4 Reasons to Think Twice

Group A Vegetables Linked to Can.cer Experts Warn to Stop Eating Them Immediately

“The Poor Shouldn’t Buy a House on the 2nd Floor, and the Rich Shouldn’t Live on the 18th”

Take Charge of Your Health – Exercise Your Way to a Can.cer-Free Future!

Discover the Secret Omega-3 Nut That Can Naturally Lower Bloo.d Lipids & Improve Heart Health

The Power of Walnuts: A Superfood for Kid.ney Health and Brain Function

Keep Your Kid.neys Healthy with These Simple, Natural Choices

Reduce Your Can.cer Risk with These Simple Food Choices

Experts Say These Four Foods Could Be Part of the Reason. Smart People Have Already Given Them Up

3 Danger.ous Ways Eating Red Dates Could Ha.rm Your Health
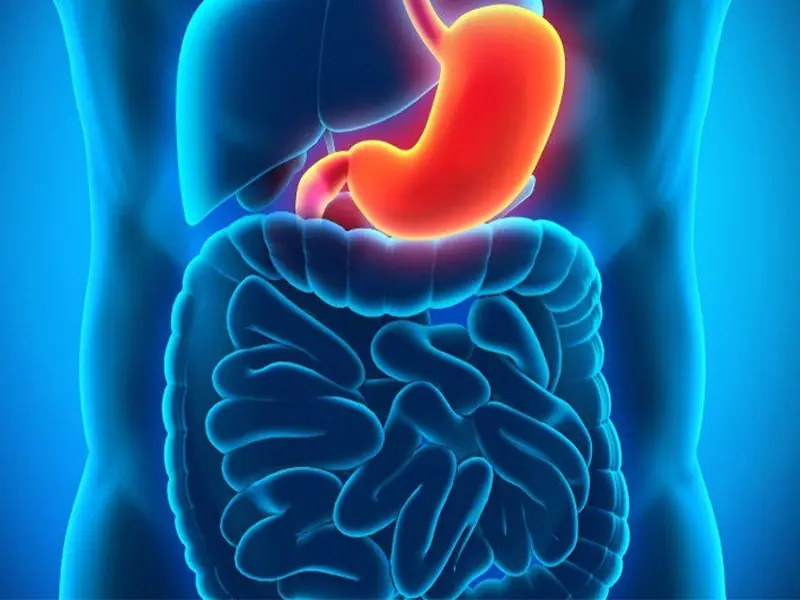
Stop Eating These 3 Foods That Are Secretly Des.troying Your Sto.mach Health

5 Natural Drinks to Keep Your Li.ver Healthy and Detoxified

This ‘Super Fruit’ Could Be the Secret to Health, Beauty, and Youth

How Eating Yogurt Before Bed Can Transform Your Health

Can.cer Will Be Defeated If You Adopt These 11 Powerful Habits!

Frequent Drooling During Sleep? It Could Be a Sign of These Six Health Issues

5 Natural Bloo.d Cleansing Drinks to Detoxify and Boost Circulation

Doctors warn: three distinct hand signs may indicate li.ver failure. If you notice any of them, don’t delay seeing a doctor.
