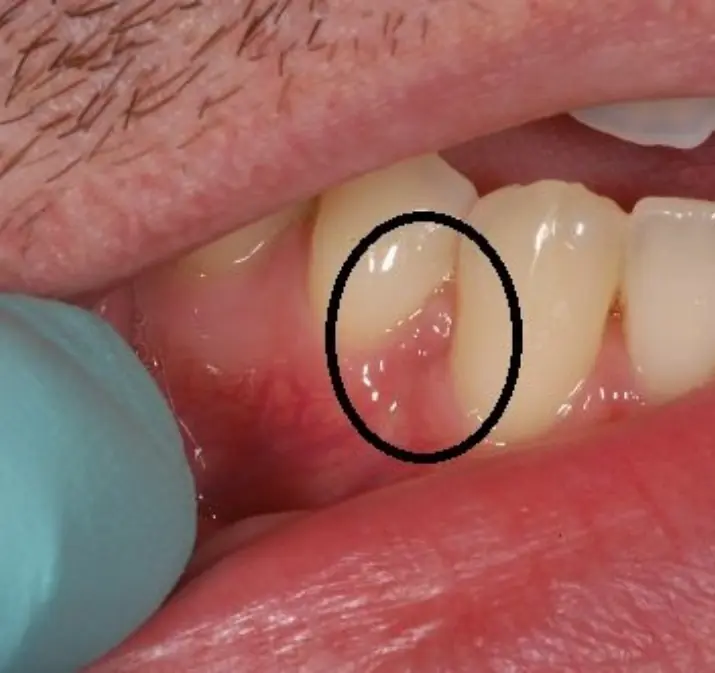
These may be the initial symptoms your gums are trying to show
Early gum changes may reveal important clues about your health.

Early gum changes may reveal important clues about your health.

A Simple Nighttime Trick: Hanging a Towel on the Door Handle and Why It Helps
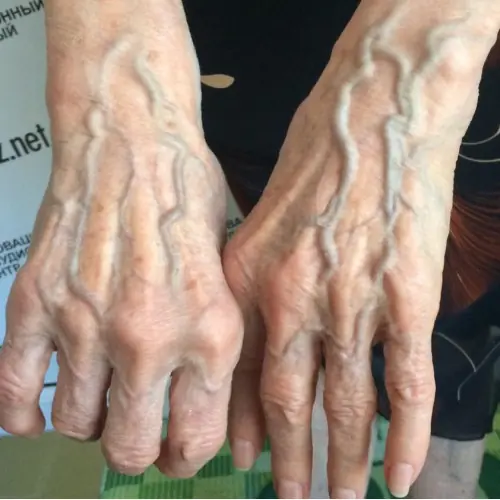
If you come across someone with bulging veins, you should definitely tell them this

Why Do You Often Wake Up Between 3 and 4 AM? 4 Possible Health Reasons

Nighttime symptoms may quietly signal early warning signs of diabetes.

Just 4 ingredients — and you can make banana vinegar at home

Nighttime Leg Cramps Could Be a Sign Your Body Needs Attention
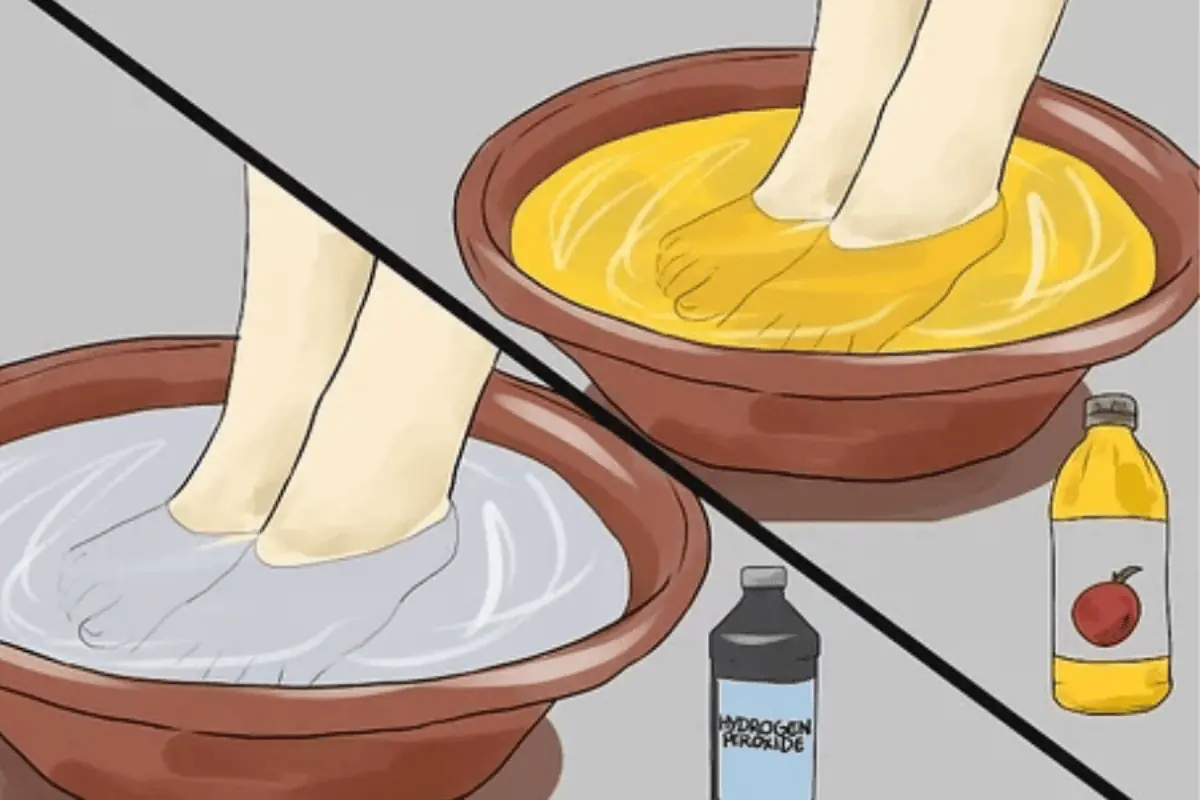
Apple cider vinegar has long been used as a natural home remedy for many everyday health concerns. Recently, one simple wellness habit has gained attention online: soaking your feet in apple cider vinegar.

The Reason Some Hotels Use Transparent Glass Doors for Bathrooms

Simple DIY trick helps fix moldy or peeling walls quickly at home.

Doctors reveal surprising effects of eating hard-boiled eggs regularly
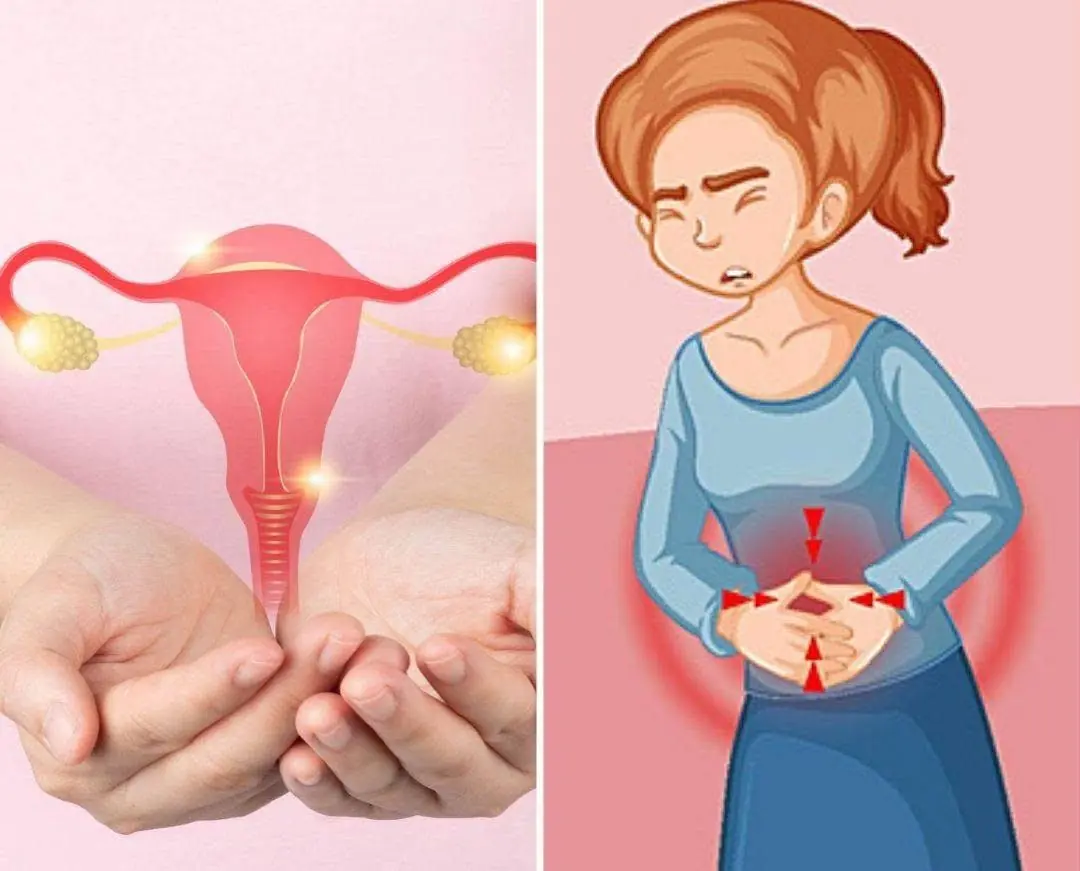
8 Early Warning Signs Of Ovarian Can:cer You Shouldn’t Ignore..

A simple natural trick can make laundry brighter and cleaner.
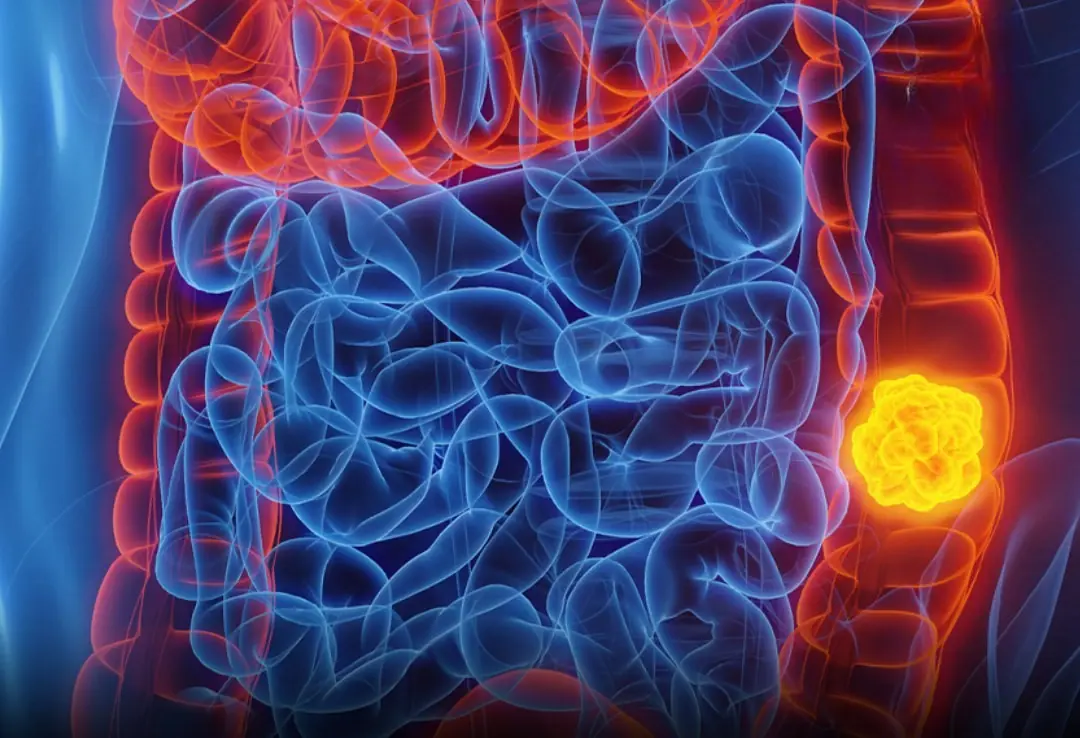
Woman with Stage - Four Colon Can:cer Reveals 5 Symptoms She Overlooked
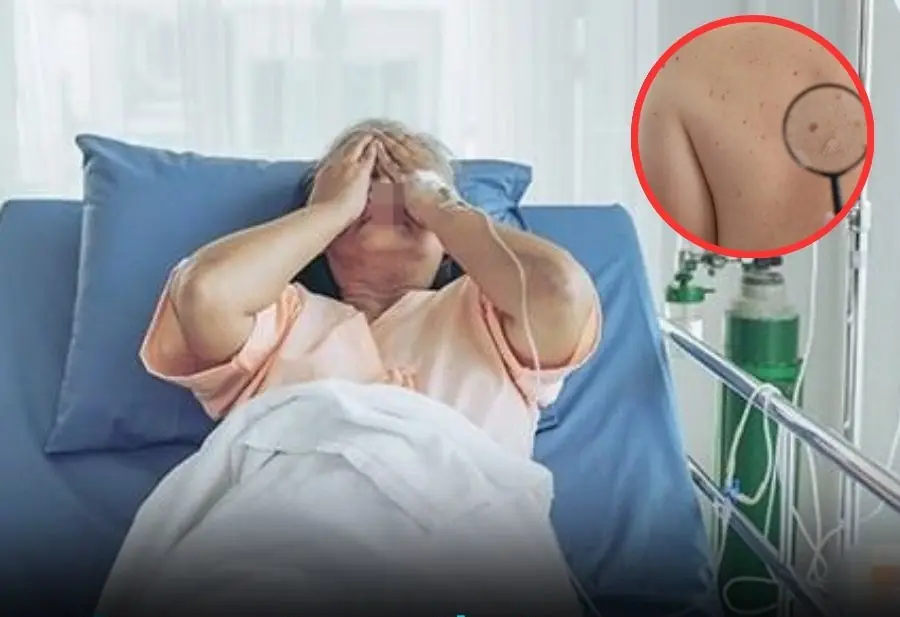
These 6 warning signs often appear late

Frequently waking up at 3 or 4 a.m. can be linked to underlying health or lifestyle factors
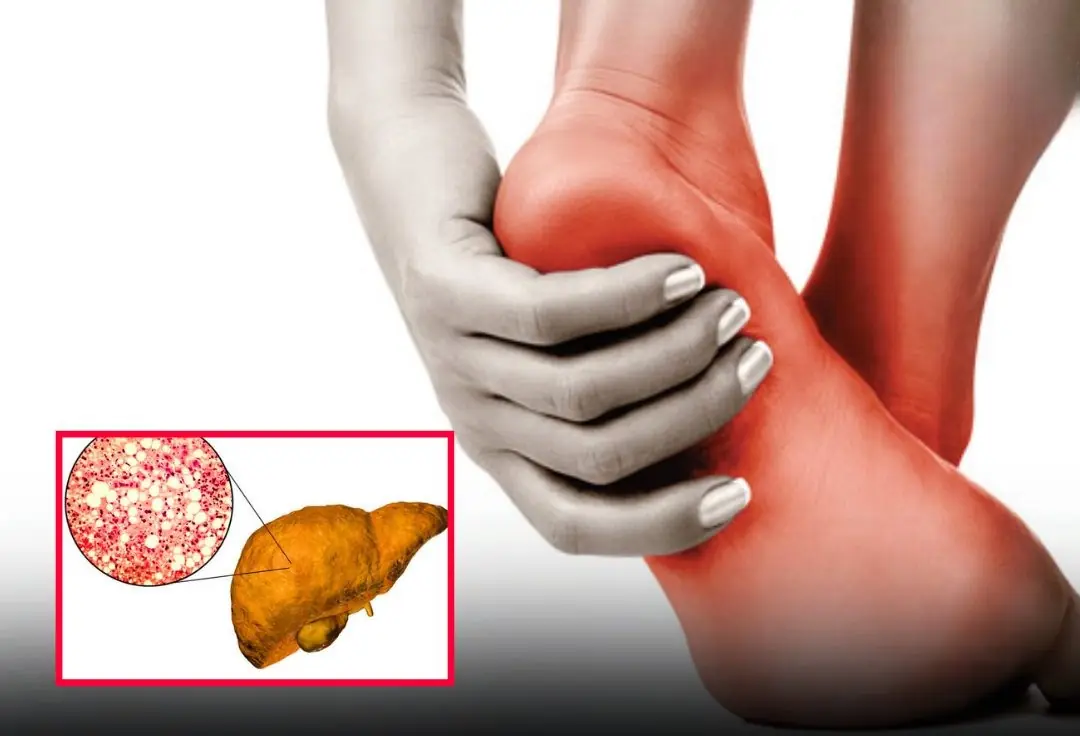
A change in your feet may signal severe fatty liver disease — don’t ignore it
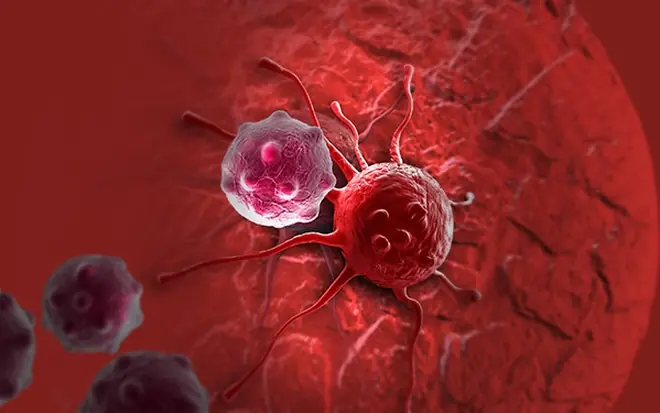
Why Can.cer Rates May Be Rising and 6 Vegetables That Contain Natural To.xins?
Here’s a quick test you can do yourself

Waking Up With a Dry Mouth at Night? Here Are 8 Possible Reasons