Recipients of the Johnson & Johnson COVID-19 vaccine in San Francisco will be able to request a dose of the Pfizer-BioNTech or Moderna Covid vaccine.
The San Francisco Department of Public Health (SFDPH) and Zuckerberg San Francisco General Hospital made the announcement on Tuesday.
The SFDPH said that it decided to make the decision after receiving requests from residents to get a second shot for extra protection against COVID-19.
The Centers for Disease Control and Prevention (CDC) is hoping to track down and record these types of shots – which they have not yet approved.

San Francisco residents who received the Johnson & Johnson vaccine will be allowed to request a ‘supplemental’ dose of the Pfizer or Moderna shots, Pictured: Carl Champion receives the Moderna COVID-19 vaccine in San Francisco, California, April 2021
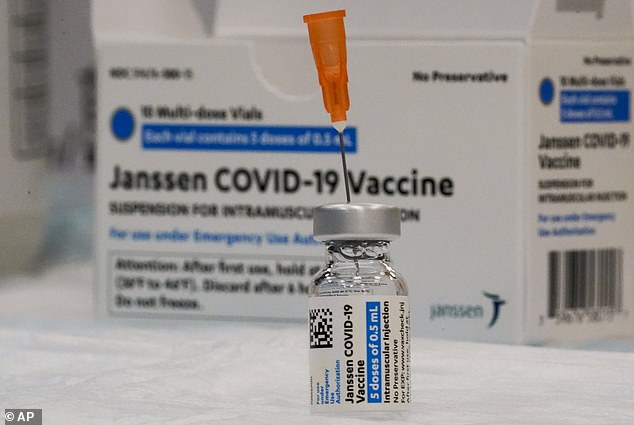
The Johnson and Johnson vaccine is believed to be effective at combatting all strains of COVID-19, including the Delta variant. Pictured: a vial of the Johnson & Johnson vaccine
‘We have gotten requests based on patients talking to their physicians, and that’s why we are allowing the accommodations,’ said Naveena Bobba, deputy director of health at SFDPH, per CNBC.
San Francisco officials do not want to refer to these shots as booster shots, instead referring to them as ‘supplemental doses’.
The CDC does not yet recommend Americans get boosters – or additional COVID-19 shots, though officials are discussing the potential of approving third doses in the near future.
Taking matters into your own hands, and getting a third dose before approved, is not recommended by the U.S. Food and Drug Administration.
Mixing different COVID-19 vaccines with one another is also not recommended by federal health officials at this time.
However, the practice has been adopted in another countries, such as Thailand and Canada, despite the lack of data on long-term effects.
The CDC has launched trials to investigate the matter, though.
The SFDPH says that its decision is not a deviation from CDC recommendations.
‘This move does not represent a change in policy for SFDPH,’ the agency said in a statement.
‘We continue to align with the Centers for Disease Control and Prevention guidance and do not recommend a booster shot at this time. We will continue to review any new data and adjust our guidance, if necessary.’
The Johnson & Johnson vaccine is the only one dose Covid vaccine available in the United States.
While the Johnson & Johnson vaccine has been maligned by some in comparison to the other two vaccines available, it is believed to be effective against the Indian ‘Delta’ variant that is causing case surges across the country.
At the start of July, J&J published data showing their vaccine was effective at preventing complications from the variant, even eight months after receival.
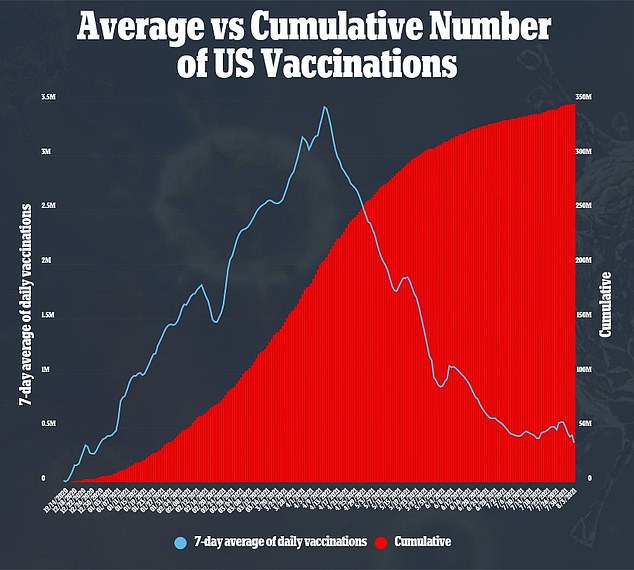
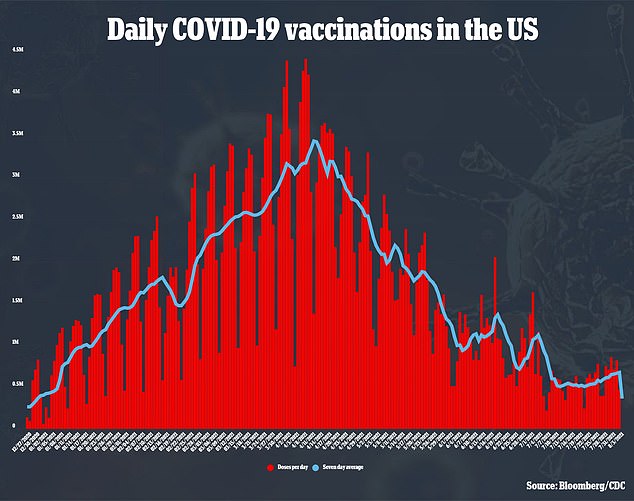
Many are seeking out third doses anyways, though, even lying about being fully vaccinated to get jabbed once again.
Dr Rochelle Walensky, director of the CDC, said earlier this week that her agency has the ability to find these incidents in their data.
The U.S. is currently sitting on a large surplus of vaccines, leading to many providers not being shrewd with their supply.
Some experts recommend they begin checking vaccine records to avoid giving someone an unapproved COVID vaccine dose.
“Reviewing a person’s history before administration has been the best practice for many years,” Rebecca Coyle, executive director of the American Immunization Registry Association, told STAT News.
“It’s time for a conversation about “Maybe I should do that,” as it hasn’t been on everyone’s minds yet.”
Health insurers, which are billed by providers when a someone they cover is vaccinated, may also have a right to refuse the bills since they are not approved.
It is unsure whether these costs will be passed over to the patient instead.
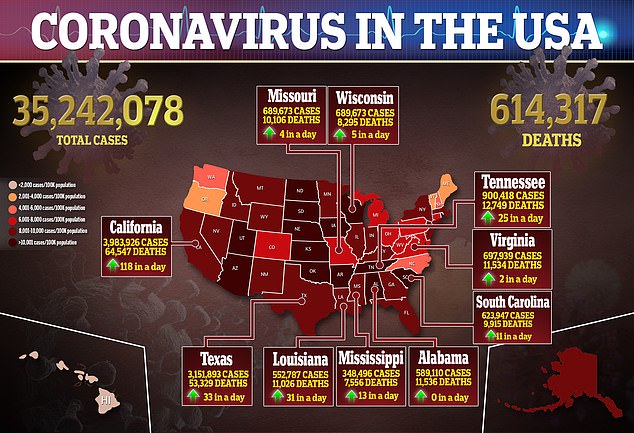
Source link : https://www.dailymail.co.uk/health/article-9861253/San-Francisco-says-Johnson-Johnson-recipients-extra-dose-Covid-vaccines.html











