Johnson & Johnson announced on Wednesday that a second dose of its COVID-19 vaccine generates a strong immune system response.
People who received a second shot of the vaccine six to eight months after their first shot saw antibody levels nine-fold higher than those who received a single dose.
The New Jersey-based company says the data provide evidence that J&J recipients should receive booster shots eight months after their initial vaccination.
It comes after the White House announced plans to roll out booster shots for people who receive the two-dose Pfizer-BioNTech and Moderna vaccines.
Johnson & Johnson said data shows that people who receive a second shot of its COVID-19 vaccine see antibody levels increase nine-fold (file image)
The firm says this is evidence that J&J recipients should be given booster shots eight months after receiving the first dose
In a press release, J&J said there were large increases in antibody levels among participants between ages 18 and 55 and in those 65 and older who were given a lower booster dose.
Researchers did not specify how many participants were given booster shots or where the studies were conducted.
However, according to information posted to clinicaltrials.gov, volunteers were dosed at least six months after their first shot.
The study is a follow-up to interim data published in The New England Journal of Medicine in July that found antibody levels after a single-shot remained stable for at least eight months and only fell 1.6-fold when tested against the Indian ‘Delta’ variant.
J&J said two manuscripts were sent to pre-print server medRxiv.org, but neither has been posted on the site as of Tuesday morning.
The firm adds that it plans to submit data to the U.S. Food and Drug Administration (FDA), which is currently reviewing similar data from Pfizer and Moderna.
Currently, boosters are only authorized for immunocompromised Americans, but the Biden administration announced last week that, pending FDA approval, all adults aged 18 and older who received the Pfizer or Moderna vaccine should get a booster shot.

Previous data released by J&J said antibody levels remained steady and only fell by 1.6-fold when tested against the variant (B.1.617.2) compared to the original strain and other variants
Federal officials said they had not included J&J in their initial plans for booster shot rollouts due to a lack of data.
‘We…anticipate booster shots will likely be needed for people who received the J&J vaccine,’ a joint statement from the Department of Health and Human Services and public health experts read.
‘Administration of the J&J vaccine did not begin in the U.S. until March 2021, and we expect more data on J&J in the next few weeks. With those data in hand, we will keep the public informed with a timely plan for J&J booster shots as well.’
This left J&J vaccine recipients in limbo, especially the immunocompromised who may be at risk from the Delta variant despite being fully vaccinated.
J&J hopes that it can join its rival vaccine makers when boosters shot start being distributed on September 20.
‘We have established that a single shot of our COVID-19 vaccine generates strong and robust immune responses that are durable and persistent through eight months,’ said Dr Mathai Mammen, global head of research and development at J&J in a statement.
‘With these new data, we also see that a booster dose of the Johnson & Johnson COVID-19 vaccine further increases antibody responses among study participants who had previously received our vaccine.
‘We look forward to discussing with public health officials a potential strategy for our Johnson & Johnson COVID-19 vaccine, boosting eight months or longer after the primary single-dose vaccination.’
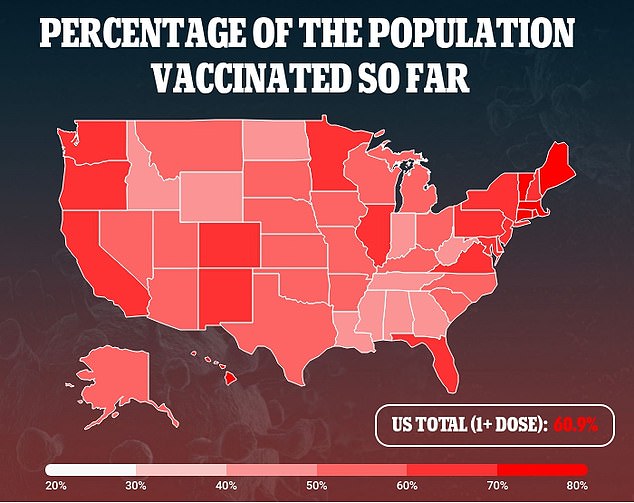
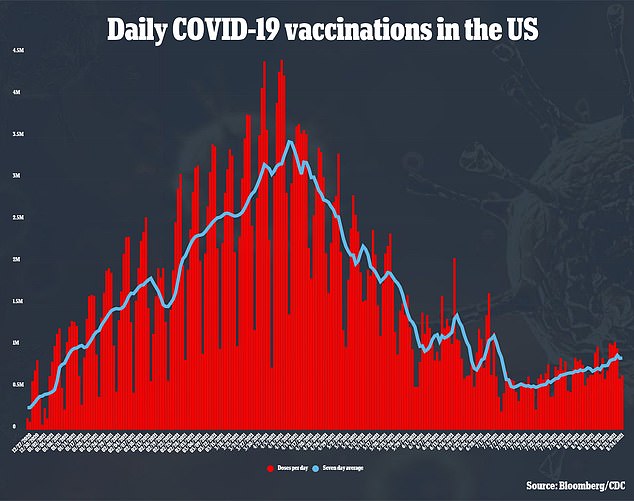
In February 2021, J&J’s shot became the third COVID-19 vaccine approved for emergency use authorization (EUA) after a clinical trial showed 72 percent efficacy against infection.
Since then, 14.1 million American have receive the single-dose vaccine, according to data from the Centers for Disease Control and Prevention (CDC).
Because the study was conducted before the highly transmissible Delta variant became widespread, officials worried that the one-shot vaccine wouldn’t offer much protection.
However, data released from a clinical trial in South Africa earlier this month found that the J&J vaccine was 71 percent effective against hospitalization from Delta and 96 percent effective against death.
It was even more effective than against the Beta variant, which originated in South Africa, joint lead investigator Glenda Gray said in a media briefing at the time.
However, J&J’s vaccine has been met with several setbacks since receiving that initial EUA.
Faith in the only one-shot vaccine available in the U.S. has faltered over the past several months.
Only weeks after approval, the vaccine’s use was paused by the FDA and CDC after cases of some recipients forming blood clots.
The pause was lifted after 11 days, but with a new blood clot warning added to the label.
Then, last month, the FDA revised the vaccine’s label, adding a warning that the shot had been linked to Guillain-Barré, a rare autoimmune disorder in which a person’s immune system attacks their own nerves.
Source link : https://www.dailymail.co.uk/health/article-9925809/Johnson-Johnson-says-second-dose-COVID-19-vaccine-raises-antibody-levels.html











