Moderna Inc announced on Thursday it is developing a single-dose vaccine that combines a booster shot against COVID-19 and a booster against influenza.
The new vaccine, which is being called mRNA-1073, will be a blend of the company’s Covid immunization with a flu shot that Moderna is developing.
‘Today we are announcing the first step in our novel respiratory vaccine program with the development of a single dose vaccine that combines a booster against COVID-19 and a booster against flu,’ Moderna CEO Stéphane Bancel said in a statement.
‘We are making progress on enrolling patients in our rare disease programs, and we are fully enrolled in our personalized cancer vaccine trial. We believe this is just the beginning of a new age of information-based medicines.’
There are currently no details of when clinical trials will begin or how soon the vaccine will be available to the general public.

Moderna announced on Thursday it is developing a one-shot vaccine that combines a booster against COVID-19 and a booster against influenza. Pictured: Moderna headquarters in Cambridge, Massachusetts, May 2020
The shot, mRNA-1073, will be a blend of Moderna’s Covid immunization with a flu shot that the firm is developing. Pictured: Volunteers are given a shot in the Moderna trial in Henry Ford Health System in Detroit, August 2020
Moderna, a one-time relatively unknown company, became a household name when it became the second firm to receive emergency approval for its COVID-19 vaccine in December 2020 for those aged 18 and older.
It is one of the first vaccines in the world – along with the Pfizer-BioNTech Covid vaccine – which uses new messenger RNA (mRNA) technology.
The technology works by using part of Covid’s genetic code to trick the body into producing a harmless piece of the virus.
This gets the body to recognize the invader and mount an immune response by making customized proteins that are ready to attack if a person becomes infected.
Shares of Moderna rose by around five percent following the announcement.
It comes after Novavax Inc said on Wednesday it has initiated an early-stage study to test its combined flu and COVID-19 vaccine.

No details are known of when clinical trials will begin or how soon the vaccine will be available to the general public. Pictured: A researcher works in a lab run by Moderna, November 2020
Participants will receive a combination of the company’s COVID-19 vaccine candidate, NVX-CoV2373, and its Influenza shot NanoFlu along with an adjuvant or vaccine booster.
NanoFlu/NVX-CoV2373 had elicited robust responses to both influenza A and B and protected against the coronavirus in pre-clinical studies.
Moderna also provided updates on Thursday regarding its ongoing trial testing its COVID-19 vaccine in children aged six months to less than 12 years.
The firm’s vaccine, which received emergency authorization for people aged 18 and older in the U.S. in December, is currently under an FDA review for use in those between ages 12 and 17.
As part of the pediatric trial, the company said it will test 50 micrograms of its vaccine in a study group of 4,000 children between six years and less than 12 years.
Moderna said dose selection studies for different age groups such as six years to less than six years, and six months to below two years, were still underway.
The company is also developing other vaccines including one that combines candidates for RSV and human metapneumovirus and another against Epstein-Barr virus.
‘I am proud of the progress that the Moderna team has made in advancing our best-in-class mRNA pipeline while addressing the global COVID-19 pandemic,’ Bancel said in his statement.
‘We believe our mRNA platform can solve the world’s greatest health challenges, from diseases impacting millions, to ultra-rare diseases impacting dozens, to medicines personalized down to the individual level.’
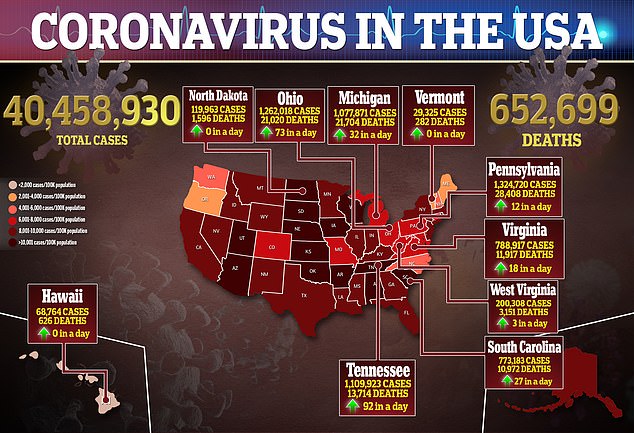
Source link : https://www.dailymail.co.uk/health/article-9973713/Moderna-developing-single-dose-booster-shot-COVID-19-flu.html











