Pfizer and BioNTech are soon planning to seek approval for their COVID-19 vaccine in children aged five to 11.
Dr Özlem Türeci, chief physician for BioNTech, told German news site Der Spiegel that the companies are set to shortly release results from their study in kids under age 12 and will ask for the shot to be approved for emergency use authorization by the U.S. Food and Drug Administration (FDA) and other agencies.
‘In the coming weeks, we will present the results of our study on the five-to-11-year- olds worldwide to the authorities and apply for approval of the vaccine for this age group,’ Türeci said.
She added that the vaccine formula is the same as that approved for adolescents and adults, but that the dose size is smaller.
Currently, the Pfizer vaccine is only approved for children aged 12 and older in both the U.S. and the European Union.
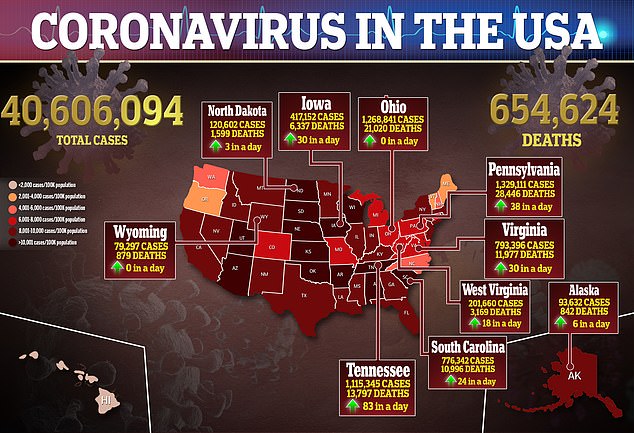
Parents and doctors have been debating about whether or not to inoculate children because they make up 0.1 percent of all Covid deaths in the U.S.
Pfizer and BioNTech are planning to seek approval of their COVID-19 vaccine in kids aged five to 11 ‘in the coming weeks.’ Pictured: A Pfizer COVID-19 vial at a mobile clinic in East Los Angeles, July 2021
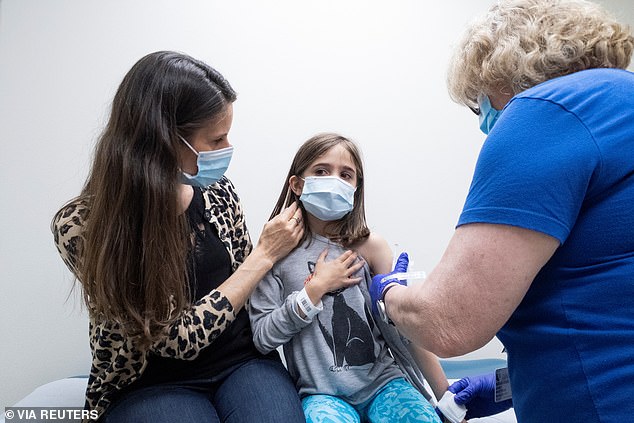
Dr Özlem Türeci, chief physician for BioNTech, said the vaccine is the same as that approved for adolescents and adults, but a smaller dose. Pictured: Marisol Gerardo, 9, is held by her mother as she gets the second dose of the Pfizer vaccine during a clinical trial at Duke Health in Durham, North Carolina, April 2021
A few hours after the new from Pfizer and BioNTech, the FDA said that clinical trial data submitted by vaccine manufacturers must include a monitoring period of at least two months after the final dose to ensure safety.
‘Children are not small adults – and issues that may be addressed in pediatric vaccine trials can include whether there is a need for different doses or different strength formulations of vaccines already used for adults,’ FDA Acting Commissioner Dr Janet Woodcock said in a joint statement with Dr Peter Marks, director of Center for Biologics Evaluation and Research.
Around 4,500 younger kids have been enrolled at nearly 100 clinical trial sites in 26 U.S. states, Finland, Poland and Spain.
According to clinicaltrials.gov, the study will work similarly to the way it did in older children and adults.
About half of the ages five-to-11 group will receive two doses 21 days apart and the other half will be given placebo shots.
The team will test the safety, tolerability immune response generated by the vaccine, by drawing blood prior to dose 1 and six months after dose 2.
If the vaccine is proven to be safe and effective, the trial will be unblinded at the six-month follow-up, meaning those who received the placebo will be allowed to get the inoculation.
Trials for kids as young as six months to four years old are still in early stages and will expand once the researchers can determine safety.
Children are often the last group to be tested during clinical trials because they are not merely little adults.
Their bodies and immune systems behave differently, meaning they might have different treatment needs.
What’s more, children may need different doses or needle sizes depending on their height, weight and age – which is why most children are only vaccinated after safety has been well-documented in the adult population.
In fact, Pfizer announced that it selected lower doses for COVID-19 vaccine trials in children than are given to teenagers and adults.
Those aged 12 and older receive two 30 microgram (μg) doses of the vaccine,
However, children between ages five and 11 will be given 10 μg doses and kids from six months to four years old will receive three μg doses.
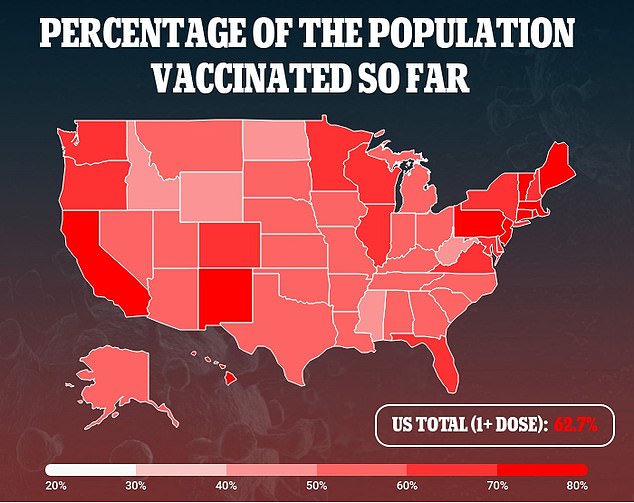
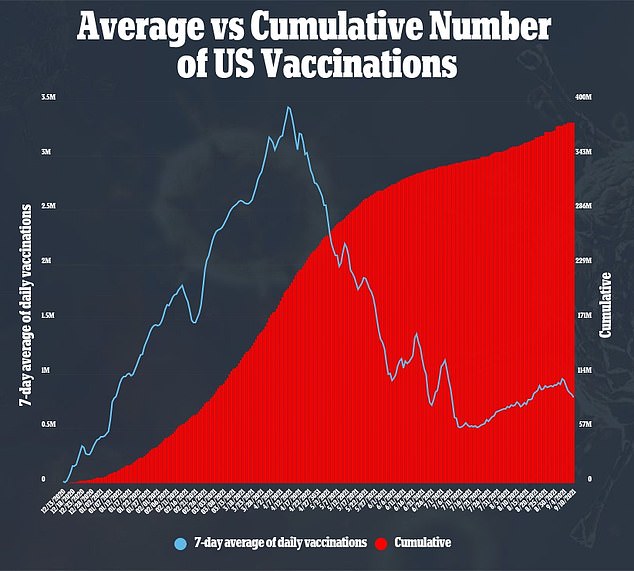
The vaccines have been proven to be highly effective in adults and teenagers, but many parents are not enthusiastic about vaccinating their children.
In April 2021 poll, conducted by the Kaiser Family Foundation, parents were asked if they would get their child immunized once a COVID-19 vaccine is authorized and available for their child’s age group.
Three in 10 parents – 29 percent – of children under 18 said they would get their child vaccinated ‘right away’ while 15 percent said they only plan to vaccinate their children if the school requires it and 19 percent said their child will definitely not be getting vaccinated.
A July 2021 survey, conducted by CS Mott Children’s Hospital National Poll on Children’s Health at Michigan Medicine last month, found that 39 percent of parents said their children already gotten a coronavirus shot.
However, 40 percent of parents also said it was ‘unlikely’ that their children would be getting vaccinated.’
According to the American Academy of Pediatrics, more than five million children have been infected by COVID-19 since the pandemic began.
However, most pediatric cases are not severe and virus-related fatalities among children are rare, with pediatric deaths making up just 0.1 percent of all COVID-19 deaths.
Source link : https://www.dailymail.co.uk/health/article-9977965/Pfizer-planning-seek-approval-COVID-19-vaccine-children-aged-five-older.html











