French vaccine company Valneva revealed today the British government had ended their Covid jab supply agreement due to a ‘breach of obligations’.
The biotech firm has been manufacturing the vaccine at its plant in Livingston, West Lothian, which Boris Johnson visited in January.
Some 100million doses of the vaccine were put on order after the UK increased its request by 40million back in February. The Government had the option of ordering an additional 90million doses to be supplied between 2023 and 2025.
But No10 has now terminated its £1.2billion agreement over allegations of a breach of the supply agreement, which the firm ‘strenuously’ denies.

Boris Johnson leaving Downing Street today after it was revealed the Government cancelled its contract with French biotechnology laboratory Valneva in Livingston
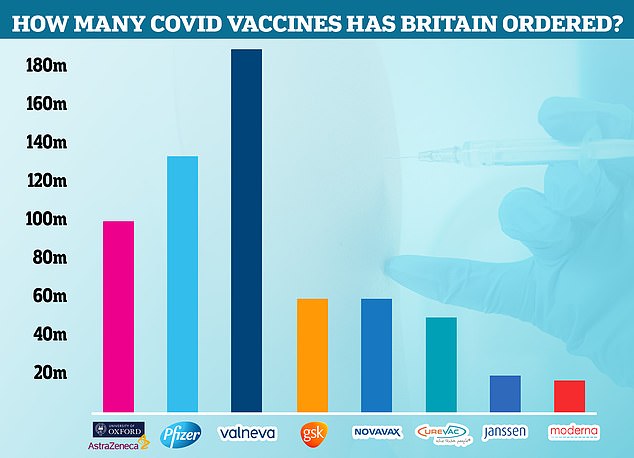
The Government cancelled its contract with Valneva for up to 190million Covid vaccines. Some 100million had already been ordered for delivery in 2021 and 2022 and the UK had the option of requesting an additional 90million that would be delivered between 2023 and 2025. Now the agreement has been terminated, Pfizer is the most-ordered jab in the UK, with 135million due to arrive in Britain by next year. Some 100million doses of AstraZeneca have been ordered, along with 60million doses each of the jabs made by GSK and Novavax. Meanwhile, the Government has requested 50million CureVac caccines, 20million Janssen and 17million Moderna injections
WHAT DO VALNEVA COVID JAB TRIALS SHOW? 
Valneva has reported the phase 1/2 clinical trial results for its coronavirus vaccine.
They involved 153 British volunteers aged between 18 and 55, who received either a low, medium or high dose of the jab given three weeks apart.
Participants were asked to report any side-effects, and had their blood tested for Covid antibodies two weeks after getting their second dose.
Does the jab trigger Covid-fighting antibodies?
Results showed everyone who received a high dose of the jab had Covid antibodies in their blood stream.
But only 89.9 per cent had them in the medium dosing group. No figure was given for those that received the low dose.
The French pharmaceutical giant said it would be advancing the high dose to phase 3 clinical trials.
Is Valneva’s vaccine safe?
No safety concerns were raised during phase 1/2, clinical trials scientists said, paving the way for it to be advanced to the next stage.
Two volunteers said they had suffered a headache or fatigue after the jabs, but these symptoms are also triggered by other vaccines already approved for use in the UK.
How does the vaccine work?
The vaccine is the only one being developed in Europe to use an inactivated whole Covid virus to trigger an immune response.
When it is injected the body attacks the spike proteins on the virus – which it uses to invade cells – by making antibodies that can bind to them, stopping an infection from happening.
The virus is killed before it is injected using chemicals, heat or radiation, meaning there is no risk of it triggering an infection.
This type of vaccine is already used to protect against polio and flu.
What is the next stage for the vaccine?
A phase 3 trial was launched in the UK last month, which involves giving over 4,000 older Britons a high dose of the vaccine.
Results from this trial and approval in the UK are expected by the end of the year, the company said.
The injection was also one of the seven Covid vaccines included in the Cov-Boost trial.
The study, led by researchers at University Hospital Southampton NHS Foundation Trust, aims to determine which jab is the most effective for booster doses.
What was Valneva’s agreement with the UK?
The UK ordered 100million doses from the French vaccine-maker to be delivered in 2021 and 2022.
The contract also allowed the UK to order an additional 90million jabs between 2023 and 2025.
But Valneva announced today that the Government terminated the contract, claiming the company breached its obligations.
Advertisement
In a statement, Valneva said: ‘Valneva SE, a specialty vaccine company, today announced it has received a termination notice from the UK Government (HMG) in relation to the Supply Agreement for its Covid vaccine candidate, VLA2001.
‘The contract provides HMG with the right to terminate.
‘HMG has alleged the company is in breach of its obligations under the supply agreement, but the company strenuously denies this.’
Neither party explained the nature of the breach.
MailOnline has approached both Valneva and the Department of Health and Social Care for comment.
Valneva’s vaccine is currently in phase three trials, with results due in the fourth quarter.
‘Subject to these data and MHRA (Medicines and Healthcare products Regulatory Agency) approval, Valneva believes that initial approval for VLA2001 could be granted in late 2021’, the company added.
‘Valneva has worked tirelessly, and to its best efforts, on the collaboration with HMG including investing significant resources and effort to respond to HMG’s requests for variant-derived vaccines.
‘Valneva continues to be committed to the development of VLA2001 and will increase its efforts with other potential customers to ensure that its inactivated vaccine can be used in the fight against the pandemic.’
Scotland’s Health Secretary Humza Yousaf said the announcement is a ‘blow’ for the facility in Livingston.
He told BBC Good Morning Scotland: ‘We are very keen, and will be reaching out to the company, to try to get security and secure a future for that facility in Livingston; we hope that would be with Valneva.
‘Clearly, when it comes to their supposed alleged failure to meet their contract obligations, we obviously are looking for more information from the UK Government and would expect that shortly.’
Britain is expected to sign off on plans to dish out booster vaccines in the coming days.
But only Covid jabs made by Pfizer and AstraZeneca have been approved to dish out as third doses currently.
None of the other vaccines ordered by No10, such as Johnson and Johnson or Moderna, have been approved as top-ups yet.
The Joint Committee on Vaccination and Immunisation (JCVI) met yesterday to consider who should get a booster shot, with a decision expected within days.
Its members are looking at the latest data from the Cov-Boost trial run by the University Hospital Southampton.
The £19.3million UK clinical trial is testing the Pfizer jab alongside those from AstraZeneca, Moderna, Novavax, Janssen from Johnson and Johnson, Valneva and CureVac.
The study is answering key questions such as whether people who have had two doses of AstraZeneca may get more benefit if they have a third dose of Pfizer.
The new guidance from the Medicines and Healthcare products Regulatory Agency (MHRA) says Pfizer boosters can be given to anyone, regardless of which doses they had previously.
However, AstraZeneca boosters will only be given to those who previously had the AstraZeneca jab.
Covid vaccinations are now estimated to have directly averted 112,300 deaths in England, according to new figures.
Previous estimates had put the number at 105,900 deaths.
Around 24.7million infections have also been prevented by the vaccine rollout, along with 143,600 hospital admissions among people aged 65 and over.
The figures, which have been calculated by Public Health England and Cambridge University, cover the period up to August 27.
Nearly 89 per cent of all people aged 16 and over in England have now received one dose of vaccine, while 80 per cent are fully vaccinated.
Vaccine take-up continues to be lower among younger age groups, however.
An estimated 83 per cent of 30 to 39-year-olds in England have now had one jab, along with just 73 per cent of people aged 18 to 29.
Separate figures from Public Health England show Covid case rates are rising in all regions of England, except the south-west.

The Biotech company has been manufacturing the vaccine at its plant in Livingston, West Lothian, and was planning on expanding the plant with 200 new jobs
North-east England has the highest rate, with 378.6 cases per 100,000 people in the seven days to September 5, up from 320.3.
London has the lowest rate at 240.0, up slightly from 237.5.
Case rates are also continuing to rise in most age groups, except for 20 to 29-year-olds, 60 to 69-year-olds and people aged 80 and over.
The highest rate is among 10 to 19-year-olds, with 681.4 cases per 100,000 people in the seven days to September 5, up sharply week-on-week from 478.3.
The lowest rate is among people aged 80 and over, at 114.0, down slightly from 115.4.
Source link : https://www.dailymail.co.uk/news/article-9984633/UK-cancels-French-covid-vaccine-maker-Valneva-breach-obligations.html











