An arthritis drug does not significantly increase a severe COVID-19 patient’s chance of survival, a new study finds.
Researchers at 39 U.S. and European hospitals investigated the drug, called canakinumab, and found a difference of just three percent in rates of survival without a ventilator between those patients who were and weren’t treated with the medication.
Overall survival and recovery rates were also similar between those patients who did and didn’t receive canakinumab.
The drug is a type of monoclonal antibody – it relies on synthetically made immune system particles.
While some monoclonal antibodies have been useful in treating COVID-19 patients, not all treatments are successful, and the team says more research is needed in this area.

A monoclonal antibody treatment used for arthritis has not shown to be effective in severe Covid patients, a new study finds
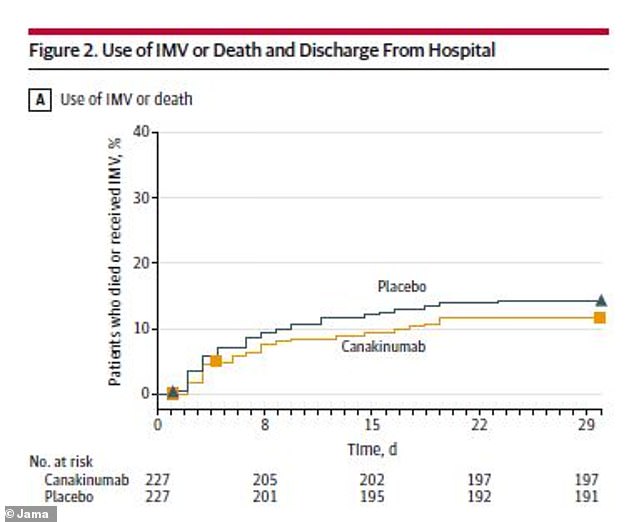
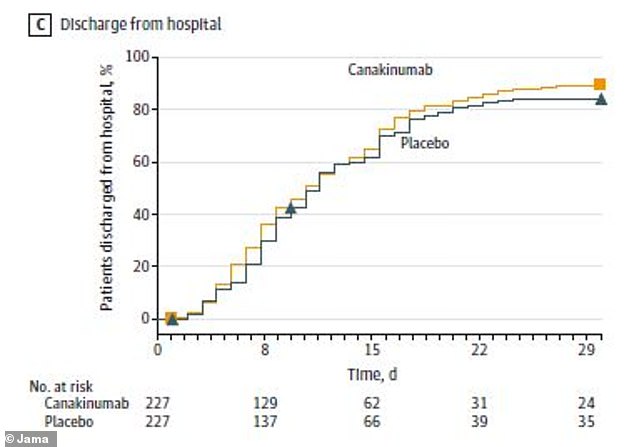
Patients who received the arthritis drug had similar chances of surviving without a ventilator (IMV) or going home from the hospital compared to those who didn’t receive it
As researchers have sought to find treatments that help save Covid patients’ lives, one common strategy has been repurposing drugs used against other diseases.
For example, the antiviral drug remdesivir was first developed to treat hepatitis C. It’s now one of the most-used treatments for hospitalized Covid treatments.
Monoclonal antibodies are another common drug type, developed in the 1980s and 1990s. T
his treatment uses artificially made antibodies – pieces of the immune system – to boost a patient’s ability to fight disease.
The Food and Drug Administration (FDA) has given Emergency Use Authorization to several monoclonal antibody treatments that target Covid.
Researchers are now investigating other, similar treatments – including canakinumab, a monoclonal antibody treatment commonly used to treat a childhood arthritis condition.
A new study from an international group of researchers found that this arthritis drug did not successfully improve chances of survival for severely ill Covid patients.
The research group included doctors from 39 hospitals in the U.S. and Europe, led by a doctor at the Lewis Katz School of Medicine at Temple University with findings published Tuesday in JAMA.
Based on lab experiments and results from other, similar monoclonal antibody treatments, the researchers had hypothesized that canakinumab would help mitigate inflammation and organ damage in severely ill Covid patients.
The researchers tested this arthritis drug through a randomized, double-blind, placebo control trial – meaning that patients were randomly assigned to receive the drug or a placebo without knowing which they got.
To be eligible for the study, patients needed to be hospitalized with a positive Covid test, Covid-related pneumonia, low oxygen levels, and inflammation.
About 450 patients participated in total. The group included half men and half women, mostly middle-aged and older.
In addition to the arthritis drug or placebo, the patients continued to receive standard Covid care at their hospitals.
The researchers monitored patients over the course of a month, then continued following up after patients went home from the hospital.
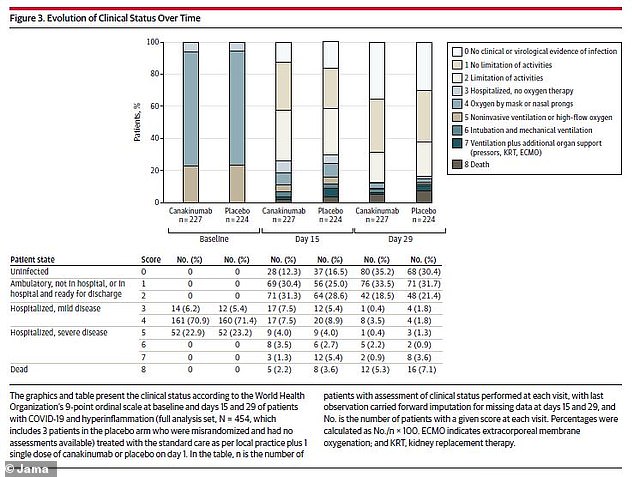
The researchers did not find significant differences in disease severity between the drug group and the placebo group over the course of the study
Within that first month, patients in the drug and placebo groups had similar chances of survival.
Out of 223 patients in the canakinumab group, 198 (88.8 percent) survived without requiring a ventilator. And out of the same number of patients in the placebo group, 191 (85.7 percent) survived without a ventilator.
That’s a difference of just 3.1 percentage points between the two groups. In order to see a significant improvement tied to the drug, the researchers hoped to see a 15 point difference.
Death and recovery numbers were also similar between the groups. In the canakinumab group, 11 patients died, while in the placebo group, 16 died.
By the end of the study month, 80 patients in the canakinumab group (35.2 percent) had no evidence of infection – the same was true for 68 patients of the placebo group (30.4 percent). Similar numbers of both groups went home from the hospital.
The researchers concluded that this monoclonal antibody didn’t significantly improve patients’ chances of survival.
Indeed, the researchers noted that a separate study of anakinra, a similar drug used to treat rheumatoid arthritis, was stopped early because that study did not find significant results.
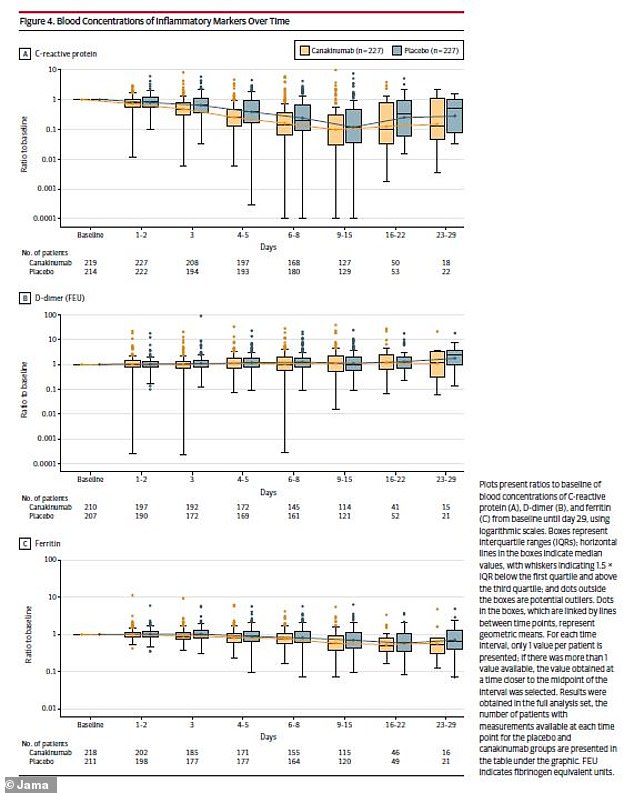
The researchers also looked at immune system markers, finding similar results between the two patient groups
Why did this trial not find that canakinumab helped Covid patients when other monoclonal antibodies have received FDA approval?
It’s possible that other treatments the patients received may have interfered with results.
Each one of the 39 hospitals involved had different treatment procedures for Covid, which were changing over the course of the study.
Additionally, experts at the National Institutes of Health recommend that, if a patient is to receive monoclonal antibody treatment, it should start as soon as that patient tests positive for Covid.
The patients in this study were already severely ill – and timing was not standardized across the different hospitals – so the treatment may have been less likely to work because it wasn’t started immediately.
In future research on monoclonal antibody treatments, more rigidly standardized trials may help researchers see conclusive results.
Source link : https://www.dailymail.co.uk/health/article-9806525/Arthritis-drug-does-NOT-increase-survival-rates-patients-hospitalized-severe-COVID-19.html











