
Probenecid (pictured), a drug used to treat gout, showed promise fighting against viruses like COVID-19 in a recent study
A drug used to treat gout could also help combat COVID-19, a new study has found.
Probenecid is medication approved by the U.S. Food and Drug Administration (FDA) for treating the condition that causes tenderness and pain at the joints.
Research published by a team from the University of Georgia finds the drug may also be able to inhibit the replication of virus cells like SARS-CoV-2 – which causes Covid – and prevent infection.
There are currently limited treatments available for the virus, and many non-virus treating drugs have been studied as potential treatments.
The drug works by preventing virus cells from taking over a person’s cells and using them to replicate.
This prevents the virus from being able to spread throughout the body and therefore limits the odds of a person falling severely ill.
There are not many drugs on the market with the ability to do this, with the ones that are currently used for Covid more effective after infection.
Researchers, who published their findings in the journal Nature, believe probenecid can combat other viruses, such the common flu, as well.
‘There’s really nothing out there to safely fight these viruses,’ said lead author Dr Ralph Tripp, a professor in the department of infectious diseases at the University of Georgia, in a statement.
‘This antiviral works for all RNA respiratory viruses we tested, including SARS-CoV-2. RSV, coronavirus and flu all circulate in the same season. Bottom line is you can potentially reduce infection and disease using this one oral drug.’
Many of the drugs used in hospitals for COVID-19 treatments are monoclonal antibodies.
The drugs are controversial, because some doubt their effectiveness, and are often not used until a person is in critical condition and hospitalized.
Researchers hope probenecid can be used early on in the process of infection, well before a Covid patient might fall seriously-ill with the virus.
‘These treatments have seen some effectiveness against SARS-CoV-2, but they’re very expensive and very hard to come by,’ Tripp said.
‘In reality, there are only a handful of options that can actually be used because of the cost, restricted IV usage, and lack of access. That’s not very useful to the world.’
Probenecid received FDA approval in 1979 and has been commonly used for treating gout patients since.
The University of Georgia researchers report the drug has limited side effects, and should be safe to use.
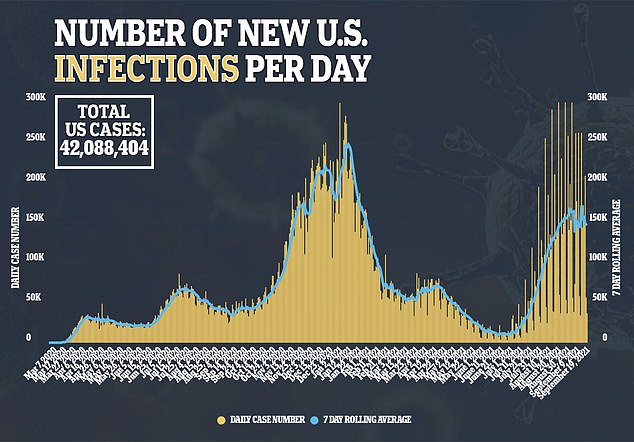
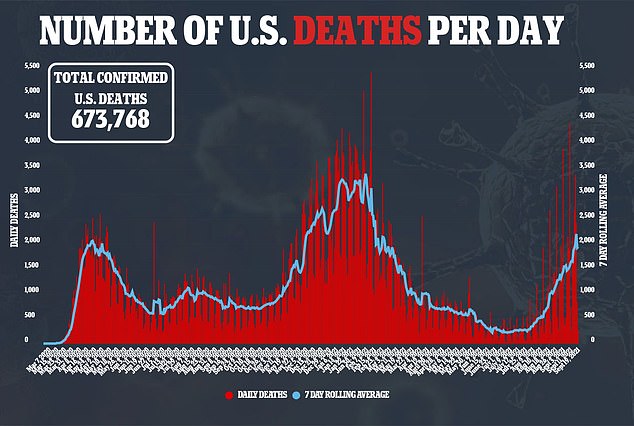
A variety of drugs that have received FDA approval for non-virus conditions have also been tested for effectiveness in combatting COVID-19.
Ivermectin, an anti-parasite drug, has recently garnered public attention after some believed it could treat the virus following a study found it could inhibit virus replication – just like probenecid.
Health experts are skeptical of the drug’s ability to treat viruses in the real world, though, as the concentrations used in the study are too high to be safe for people.
The University of Minnesota has recently launched a trial to test ivermectin’s effectiveness fighting Covid, along with a diabetes and a obsessive compulsive disorder drug.
If proven effective, these drugs can have an impact on the medical world even beyond the current pandemic.
Generic versions of these medications can be cheaply produced and distributed around the world, allowing lower income nations to easily access these potentially effective treatments for a variety of viruses.
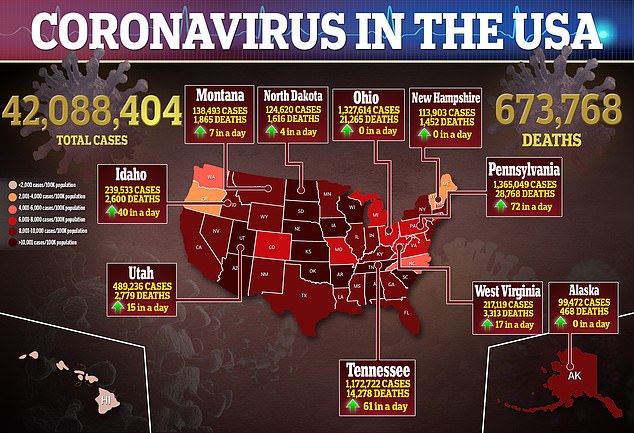
Source link : https://www.dailymail.co.uk/health/article-10010119/FDA-approved-gout-medicine-Probenecid-shows-promise-fighting-viruses-like-COVID-19.html











