The U.S. Food and Drug Administration (FDA) is planning to allow Americans with compromised immune systems to get a third COVID-19 vaccine dose from tomorrow.
Sources tell NBC News that the federal health agency will expand the emergency use authorizations for the Pfizer-BioNTech and Moderna vaccines to allow them to be administered as booster shots.
As many as three percent of all Americans are considered immunocompromised due to cancer treatment, autoimmune diseases, HIV or other ailments.
In the past few months, several studies have suggested that immunocompromised people don’t have as much protection after being fully vaccinated as healthy people.
The FDA is planning to allow Americans with compromised immune systems to get a third COVID-19 vaccine dose on Thursday. Pictured: Grace John, gets a COVID-19 shot at a mobile vaccination clinic run in Hayward, California, February 2021
Past studies have found that, even after being fully vaccinated, people with weakened immune systems have low or undetectable antibody levels. Pictured: A vial of Moderna’s COVID-19 vaccine, April 7
Previously, health experts had said that there was no evidence to suggest that fully vaccinated Americans needed booster shots.
However, more and more research has shown that people with weakened immune systems have low or undetectable antibody levels, even after two doses.
A study in May found that all cancer patients developed fewer antibodies after being vaccinated compared to healthy participants and 10 percent barely developed antibodies at all.
Another study in June looked at 30 organ transplant recipients and found that 24 developed negative antibody levels – meaning they did not have any immune-fighting cells – after two doses of the Pfizer-BioNTech or Moderna vaccines.
The findings are worrying because immunocompromised people are already at an increased risk of hospitalization or death from the virus.
This makes COVID-19 immunity even more crucial for this population.
However, third doses may be a way to boost antibody levels.
For example, the study about organ transplant patients found that one-third of patients with negative antibody levels from the first two doses now showed an increase after a third dose.
The biggest increase seen were antibody levels that rose 687-fold.
Last month, the CDC’s Advisory Committee on Immunization Practices said the agency is working to make additional doses available for immunocompromised people.
‘I think what you’re asking about is, you know, is there a way to offer a third dose to individuals…through a study, or through an investigational new drug format for this population?’ Amanda Cohn, the CDC’s chief medical officer for vaccine policy, told the panel.
‘I will just say that we are actively looking into ways that could be done to potentially provide access earlier than any potential change in regulatory decisions.’
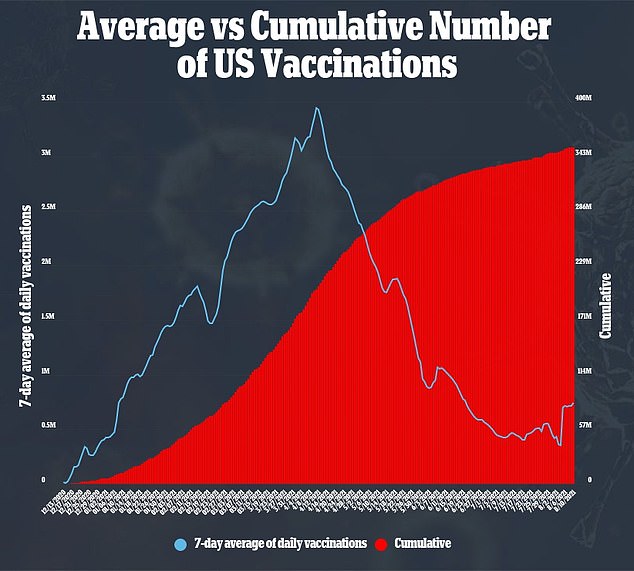
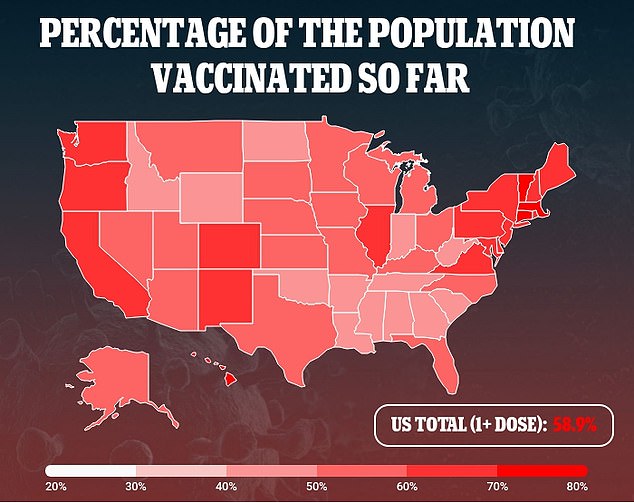
ACIP cannot recommend third doses until the FDA fully approves the vaccines or extends emergency use to include boosters.
However, ACIP is meeting on Friday and will likely vote on whether or not to recommend boosters.
During a press conference last week, Dr Anthony Fauci said that people with weakened immune systems are ‘vulnerable’ to COVID-19 infection and that the Biden administration was working to get COVID-19 vaccine booster shots approved for immunocompromised Americans.
They ‘do not make, in general, an adequate response [to the COVID-19 vaccine] that we feel would be adequately protective,’ he said.
‘It is extremely important for us to move to get those individuals their boosters, and we are now working on that and will make that be implemented as quickly as possible, because for us and, for the individuals involved, it is a very high priority.’
Third doses are currently approved in several countries including Chile, France, Germany and Israel.
Source link : https://www.dailymail.co.uk/health/article-9885203/FDA-plans-allow-immunocompromised-Americans-dose-Covid-vaccine-TOMORROW.html











