The U.S. Food and Drug Administration (FDA) remains skeptical of COVID-19 vaccine booster shots ahead of an advisory committee meeting on Friday despite Pfizer-BioNTech releasing new data suggesting the need for them.
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet at the end of the week to discuss data on potential third doses.
Members will vote on whether or not booster doses are safe and effective and if they should be approved for all Americans.
Currently, third doses are only approved for immunocompromised people in the U.S. aged 12 and older.
New data from Pfizer published on Wednesday suggests that efficacy of the regular two-dose regimen declines from 96.2 percent to 83.7 percent after six months, but that a booster dose increased immune responses.
However, in a separate briefing document also published Wednesday, FDA officials expressed doubt about the need for extra doses.

Documents ahead of an FDA advisory committee about COVID-19 vaccine booster shots were published on Wednesday. Pictured: A nurse administers a COVID-19 booster shot to Lana Sellers in Altamonte Springs, Florida, August 18
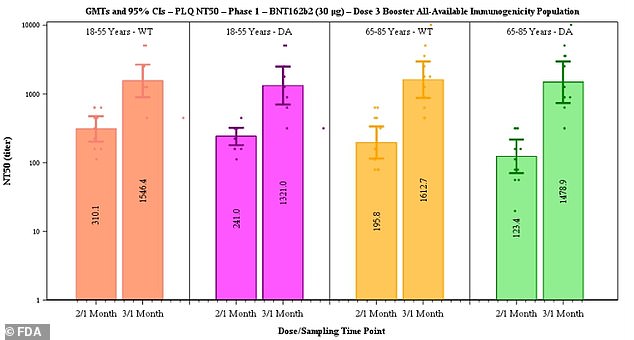
Pfizer released new data suggesting efficacy of two doses declines from 96.2% to 83.7% after six months but that third doses appeared to boost antibody levels (above) between five-fold and 12-fold, particularly among older adults
Last month, boosters were approved for immunocompromised Americans who had received either the Pfizer or Moderna vaccine after data showed they were less likely to develop high antibody levels after two doses.
At the time, Pfizer said its early data suggested people who received booster doses between six and 12 months after their final dose had high levels of protection.
The company filed for emergency use authorization for booster doses in late August and submitted data, made public on Wednesday.
The documents suggest that protection from two doses of the Pfizer vaccine declines from 96.2 percent at seven days after dose 2 to 90.1 percent two months later to 83.7 percent up to six months later.
What’s more, they cited data from Israel showing people fully vaccinated in January 2021 had a 2.26-fold increased risk for breakthrough infections compared to those fully vaccinated in April 2021.
Another Israeli study noted in the documents showed that effectiveness against infection was 39 percent and against symptomatic disease was 40 percent from June 20, 2021 to July 17, 2021, when the Delta variant was the dominant strain.
Comparatively, between January and April, these rates were at 95 percent or higher.
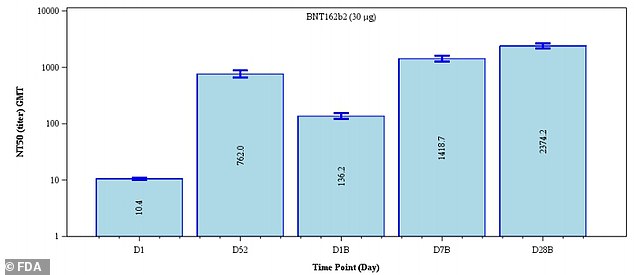
Antibody levels appeared to increase 3.1-fold 28 days after dose three (far right) compared to day five after dose 2 (second from the left)
The researchers say that even though Delta was the dominant strain during the summer, the declining percentages are a sign of waning vaccine efficacy not the variant.
‘As described…emerging data suggest that vaccine protection may wane approximately six to eight months following the second dose, and evidence is building to suggest that administration of booster doses of COVID-19 mRNA vaccines is potentially an urgent emerging public health issue,’ the authors wrote.
The team also released data a clinical trail involving 23 participants who participated in Pfizer’s early-stage trials last year.
Each had received two doses of the vaccine and were given a booster dose at least six months later.
Of the participants, 11 were in the younger adults group of those aged 18 to 55 and 12 were aged 65 to 85.
After the third dose, neutralizing antibodies against the original strain of the virus rose five-fold in the 18-to-55 age group and seven-fold in the 65-to-85 group.
Against the Delta variant, antibody levels after a booster shot rose five-fold in the younger adult group and 12-fold in the older adult group.
In a separate document, FDA scientists wrote with a skeptical tone about the need for booster shots.
‘Overall, data indicate that currently US-licensed or authorized COVID-19 vaccines still afford protection against severe COVID-19 disease and death in the United States,’ the scientists wrote.
They added that studies on booster doses have presented conflicting findings and that ‘known and unknown biases that can affect their reliability.’
Additionally, the scientists pondered over whether or not a third dose would increase rates of rare heart inflammation – myocarditis and pericarditis – seen in young males after two doses.
‘It is currently not known if there will be an increased risk of myocarditis/pericarditis or other adverse reactions after a booster dose,’ of the Pfizer vaccine, they wrote.
‘These risks and associated uncertainties have to be considered when assessing benefit and risk.’
Source link : https://www.dailymail.co.uk/health/article-9994315/FDA-remains-skeptical-booster-shots-despite-new-Pfizer-data-suggesting-efficacy-declines.html











