British Covid patients won’t yet get a ‘game-changing’ pill which halves the risk of being hospitalised or dying from the illness, it was revealed today.
US drug giant Merck today announced the landmark results of a trial of its antiviral molnupiravir.
Hospitalisation and death rates were twice as high among infected patients given a placebo, compared to those given the actual pill.
But there are no plans to make it immediately available in the UK, though Merck said it is committed to getting approval for its use in Britain. The US has already agreed to purchase 1.7million courses of the drug.
Merck’s chief science officer Daria Hazuda told a press briefing of health and science reporters that the results were an exciting step in the global fight against Covid. She described it as ‘game-changing’.
Molnupiravir works by disrupting the Covid virus’s ability to reproduce in the human body.
‘It gets incorporated into the genetic material of the virus and introduces errors,’ she said. Over time, the errors make the virus less able to replicate.
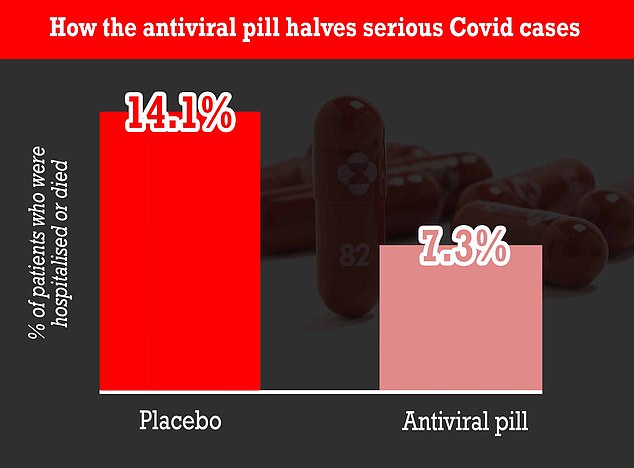
Covid patients who took molnupiravir were half as likely to die or need hospitalisation compared to the placebo group, according to the results of the Merck’s latest trial of the experimental pill. Only 7.3% of the patients who took the molnupiravir pill either died or required hospitalisation, compared to 14.1% of patients either dying or requiring hospitalisation among the patients who took the placebo pill. This means that Covid patients who took molnupiravir halved their chance of dying

American company Merck & Co today announced the results of a trial on Covid patients of its antiviral pill molnupiravir
In comparison to other anti-Covid drugs which need to be administered by medics, molnupiravir could be taken by patients at home.
Dr Hazuda also said molnupiravir could also have potential in fighting other viruses, similar to Covid, and the inevitable next pandemic.
Drugs proven to work in the fight against Covid
Budesonide
Brand name: Pulmicort
Manufactured by: AstraZeneca, Orion Pharma and Mylan
Who it is given to: over-65s or at risk over-50s
What studies showed it could do: Experts at the University of Oxford found that in two weeks, 32 per cent of people recovered from Covid when taking the drug through an inhaler, compared to 22 per cent without it. Budesonide is a corticosteroid, which means it reduces swelling inside the body and helps control immune reactions
What it costs: around £15 for one inhaler
Dexamethasone
Brand name: Ozurdex and Baycadron
Manufactured by: Companies including Aspen and Hikma Pharmaceuticals
Who it is given to: Some patients hospitalised with the virus
What studies showed it could do: A trial by the RECOVERY group found that the cheap steroid can prevent death in one in eight ventilated coronavirus patients and one in 25 on breathing support. The steroid prevents substances in the body from being released that cause inflammation, which makes breathing difficult in Covid patients. It is given as either an injection or a daily tablet
What it costs: around £5 per patient
Tocilizumab
Brand name: RoActemra and Actemra
Manufactured by: Companies including Roche
Who it is given to: some hospitalised Covid patients
What studies showed it could do: A RECOVERY trial found that arthritis drug cut the risk of death by an extra four per cent, on top of the 20 to 35 per cent reduction given by dexamethasone. This means one extra life could be saved for every 25 people given the drug. It was also found to cut the time spent in hospital by five days. It prevents a reaction in the lungs and airways that leads to respiratory issues. It is given to patients through an injection into the veins and lasts four weeks at a time
What it costs: A typical 480mg dose costs £614.40
Advertisement
‘It’s active across multiple coronaviruses, influenza and several other RNA viruses,’ she said.
‘We’re truly excited about the results, not just because of the potential for this to play a role in the current pandemic.
‘Even potentially for preventing future pandemics and future outbreaks from other coronaviruses, which I’m sure will happen.’
If approved, it will be added to the arsenal of drugs already available to NHS doctors.
Dexamethasone was the first to be given the green light last June, but it is only given to patients already ill in hospital.
And tocilizumab, which is given through an injection, is also only given to hospitalised patients.
But budesonide, a drug taken as an inhaler, can be prescribed to infected over-65s or over-50s who are at risk of the virus.
UK doctors hailed the results of the trial as a breakthrough in the fight against Covid, though some have also urged caution until more detail is revealed.
UK Bioindustry Association chair Ruth McKernan said preventing Covid infection in the first place through vaccination was the priority. But she added an oral anti-Covid pill would be a valuable weapon.
‘In order to protect people and the NHS to the maximum level possible we need all the tools at out disposable and it’s great to have the first oral drug with really high quality data,’ she said.
‘We need a full set of treatments from preventative vaccines, oral treatments for those infected and additional drugs for hospital use.’
Dr Simon Clarke, a cellular microbiologist at the University of Reading, described the trial results as promising.
‘Although the trials data are yet to be peer-reviewed, the claimed 50 per cent reduction in hospitalisation and death in early-stage infections would be impressive,’ he said.
However, Dr Clarke said more detail on the potential side affects of the experimental drug was needed.
The head of the Britain’s antivirals taskforce, Eddie Gray, declined to comment on talks to get the drug rolled out in the UK.
It is unclear how much the drug would cost the NHS if it is approved in the UK, but the US Government has bought millions of couses of the drug for $706 (£520) per patient.
‘We are involved in looking closely at all of the options available, but we’re really not in a position to give out the details around specific conversations at this moment in time,’ he told reporters today.
Pressed for more details Mr Gray said: ‘The emergence of phase three data tends to accelerate all processes of this type, but I couldn’t give you a specific date.’
‘I have chosen to turn up today to be here, read into that what you will,’ he added coyly.
Merck’s chief science officer Daria Hazuda said she though the results of the latest molnupiravir trial were ‘game-changing’

The head of the No.10’s antivirals taskforce Eddie Gray refused to be drawn on if the UK was in talks to the molnupiravir drug to Britain
The trial tracked 775 adults with mild-to-moderate Covid who were considered higher risk for severe illness due to health problems such as obesity, diabetes or heart disease.
Among patients taking molnupiravir, 7.3 per cent were either hospitalised or died at the end of 30 days, compared with 14.1 per cent of those getting the dummy pill.
There were no deaths in the drug group after that time period compared with eight deaths in the placebo group.
The results were released by the company and have not been peer-reviewed, but Merck says it plans to present them at a future medical meeting.
An independent group of medical experts monitoring the trial recommended stopping it early because the interim results were so strong.
‘It exceeded what I thought the drug might be able to do in this clinical trial,’ Dr Dean Li, vice president of Merck research, said.
‘When you see a 50 per cent reduction in hospitalization or death, that’s a substantial clinical impact.’
Side effects were reported by both groups in the Merck trial, but they were slightly more common among the group that received a dummy pill. The company did not specify the problems.
Earlier study results showed the drug did not benefit patients who were already hospitalized with severe disease.
Prime Minister Boris Johnson announced in April that he was assembling a team of scientists to find ways for people to recover from the virus without going into hospital because the UK must ‘learn to live with this disease, as we live with other diseases’.
Antivirals were one treatment touted by the PM, with Mr Johnson at the time stating the drugs could ‘provide another vital defence against any future increase in infections and save more lives’.
And there are hopes they will help stop the new variants making people seriously ill – mutated strains make it more likely that someone will get ill even after vaccination.
Source link : https://www.dailymail.co.uk/health/article-10049929/British-doctors-praise-Mercks-antiviral-pill-HALVES-risk-Covid-patients-hospitalised.html











