Novavax Inc said on Wednesday it has launched an early-stage study to test a combined regimen of its flu and Covid vaccine.
Research into more effective flu vaccines has been jump started during the pandemic, as much of what researchers learned about vaccines from developing them for Covid could be applied to other viruses as well.
Novavax hopes its vaccine candidate will be a crucial step to fighting both viruses in the future.
It come as the Gaithersburg, Maryland-based company also seeks full approval from the U.S. Food and Drug Administration (FDA) for its COVID-19 vaccine.
Novavax will soon begin clinical trials for a joint COVID-19 and flu vaccine regimen. The company will also seek FDA approval for its Covid vaccine soon. (File Photo)
The trial for the joint flu-Covid vaccine regimen is to be conducted in Australia.
Researchers will enroll 640 healthy adults between the ages of 50 and 70 and who have either been previously infected with the coronavirus or given an authorized COVID-19 vaccine at least eight weeks prior to the study.
Participants will receive a combination of the company’s COVID-19 vaccine candidate, NVX-CoV2373, and its Influenza shot NanoFlu along with an adjuvant or vaccine booster.
‘Combination of these two vaccines…may lead to greater efficiencies for the healthcare system and achieve high levels of protection against COVID-19 and influenza with a single regimen,’ Dr Gregory Glenn, president of Research and Development at Novavax, said in a statement.
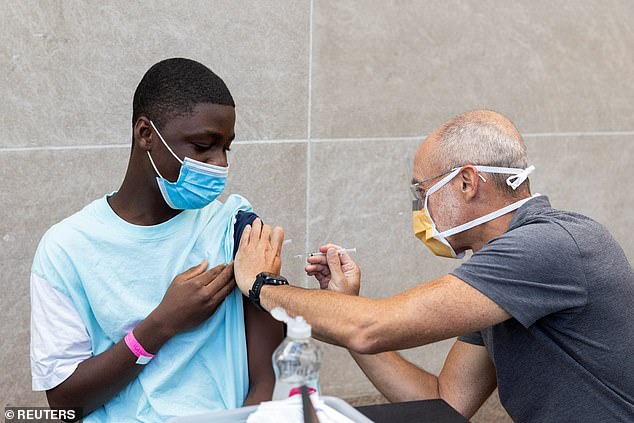
The Novavax COVID-19 vaccine is two-shots and is believed to be 90% effective at preventing infection from the virus. Pictured: A man in Philadelphia, Pennsylvania, is vaccinated for COVID-19
Novavax had said in May it expects seasonal influenza and COVID-19 combination vaccines to likely be critical in combating emerging virus variants.
Its vaccine NanoFlu/NVX-CoV2373 had elicited robust responses to both influenza A and B and protected against the coronavirus in pre-clinical studies.
Novavax expects the trial results in the first half of 2022.
The company is also planning to soon submit data for its COVID-19 standalone vaccine to the Food and Drug Administration for approval.
The vaccine is administered in two doses, just like the Pfizer-BioNTech and Moderna jabs, and showed promise in clinical trials.
Unlike those shots, though, it is not an mRNA vaccine, but instead a ‘protein subunit’ shot.
This type of vaccine has been used for decades, and uses a purified protein from the virus and inserts it into the body to trigger an immune response.
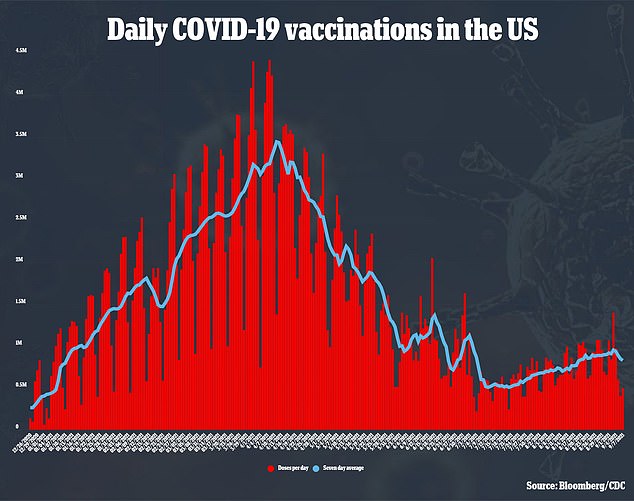
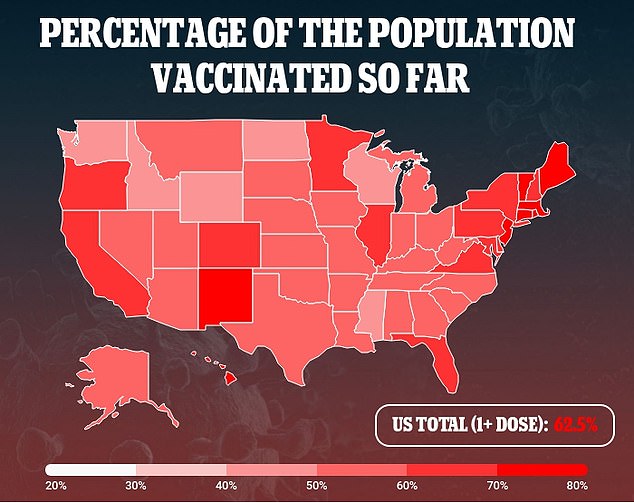
‘It’s a type of vaccine that we have used for flu, hepatitis, and HPV in the past,’Dr Mark McKenzie, regional medical director for ClinSearch, told WDEF in Chattanooga, Tennessee.
‘But this one is targeting the world. It will be available to people around the world in addition to what’s out there.
‘Hopefully it will be at a lower cost for some countries that don’t have the resources like the United States has.’
Participants who received the vaccine as part of the trial are considered fully vaccinated by the Centers for Disease for Control and PreventioN, and it believed to be 90 percent effective.
The FDA will soon review the safety of the jabs and monitor recipients for potential side-effects or other effects that may appear.
Novavax is seeking full approval for its shots, not the emergency use authorization that was initially issued for the Pfizer, Moderna and Johnson & Johnson vaccines.
The Pfizer vaccine is currently the only one that has received full approval from the FDA for all Americans over the age of 16.
Source link : https://www.dailymail.co.uk/health/article-9969689/Novavax-begins-early-stage-trial-combined-influenza-COVID-19-vaccine.html











