Pfizer Inc and its German partner BioNTech SE have asked the U.S. Food and Drug Administration (FDA) to expand emergency use of its COVID-19 vaccine to include kids between ages five and 11.
When the vaccine was originally authorized for use by the FDA in December 2020, it was only for those aged 16 and older, before being expanded to those aged 12 and older in May.
The FDA is planning to move quickly and has a meeting tentatively scheduled to discuss the matter on October 26.
Officials are expected to make a decision – which would make 28 million kids eligible – between Halloween and Thanksgiving.
Some parents are eagerly awaiting the authorization while others say they do not want to inoculate their children because of their low risk of severe illness, making up less than 0.1 percent of all Covid deaths in the U.S.
SCROLL DOWN FOR VIDEO
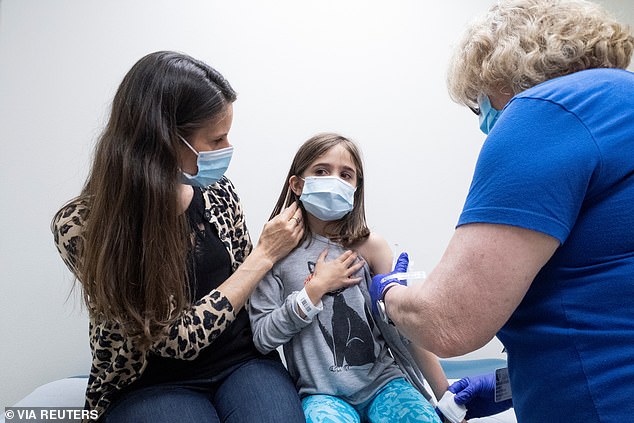
Pfizer-BioNTech asked the FDA to expand emergency use of its COVID-19 vaccine to Americans between ages 5 and 11. Pictured: Marisol Gerardo, 9, is held by her mother as she gets the second dose of the Pfizer vaccine during a clinical trial at Duke Health in Durham, North Carolina, April 2021
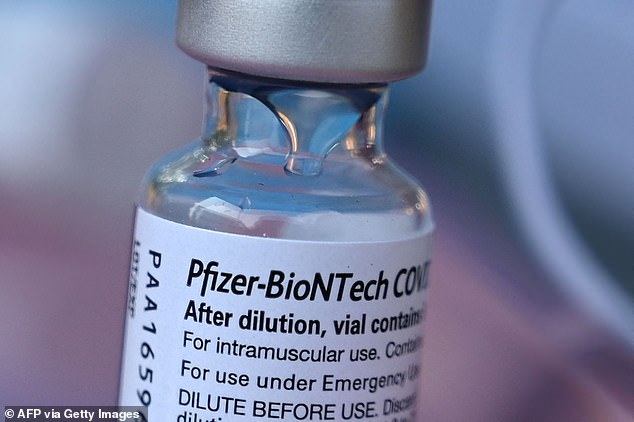
The FDA is meeting on October 26 to discuss and a decision is expected between Halloween and Thanksgiving. Pictured: A vial of the Pfizer-BioNTech at a pop up vaccine clinic in Los Angeles, August 2021
According to clinicaltrials.gov, Pfizer’s study in younger children worked similarly to the way it did in older children and adults.
A total of 4,500 younger kids aged six months and older were enrolled at nearly 100 clinical trial sites in 26 U.S. states, Finland, Poland and Spain.
Of those children, 2,268 were between ages five and 11.
About half of those in the five-to-11 group were given two doses 21 days apart and the other half were given placebo shots.
The team then tested the safety, tolerability and immune response generated by the vaccine by measuring antibody levels in the young subjects.
Pfizer said it had selected lower doses for COVID-19 vaccine trials in children than are given to teenagers and adults.
Those aged 12 and older receive two 30 microgram (μg) doses of the vaccine.
However, children between ages five and 11 were given 10 μg doses and kids from six months to four years old received three μg doses.
Unlike the larger clinical trial conducted in adults, the pediatric trial did not measure efficacy by comparing the number of COVID-19 cases among the vaccine group to the number in the placebo group.
Instead, scientists looked at levels of neutralizing antibodies in young vaccine recipients and compared the levels to those seen in adults.
The companies expect data on how well the vaccine works in children between ages two and five and between six months and two years of age by the end of the year.
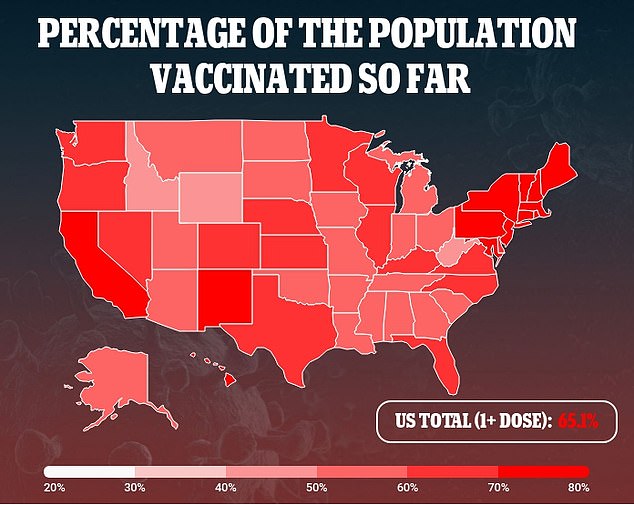
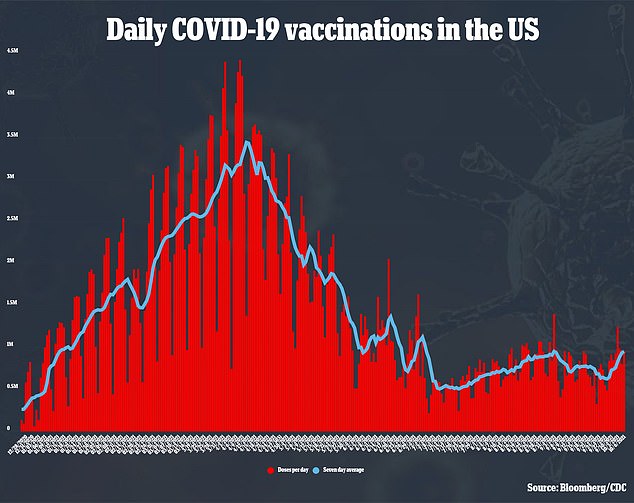
Recently, pediatric cases increased from 71,726 per week at the beginning of August to more than 243,000 in September, fueled by the Delta variant.
However, they now appear to be trending downward with 173,469 reported last week, according to the American Academy of Pediatrics.
There have also been 520 pediatric deaths since the start of the pandemic, indicating children make up less than 0.1 percent of all deaths.
Currently, no evidence suggests the Delta variant is more dangerous in kids than previous strains of the virus.
Because of this low risk of severe illness, polls have shown that many parents are not inclined to vaccinate their children.
A July 2021 survey, conducted by CS Mott Children’s Hospital National Poll on Children’s Health at Michigan Medicine last month, found that 39 percent of parents said their children already gotten a coronavirus shot.
However, 40 percent of parents also said it was ‘unlikely’ that their children would be getting vaccinated.’
Another poll from Axios/Ipsos in September found that 44 percent of parents of children aged five to 11 said their kids were likely to get a vaccine and 42 percent said it was unlikely their children would be immunized.
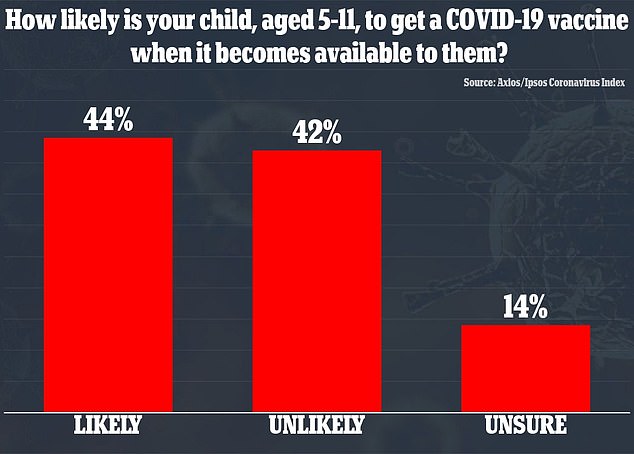
A poll from Axios/Ipsos found that 44% percent said their child was likely to get a vaccine and 42% said it was unlikely their kids would be immunized
In fact, many more children die from gun violence, drownings, poisonings and other fatal injuries each year compared to those who have died from COVID-19.
Poisoning accidents kill 730 children every year, with two deaths occurring every day, according to the CDC.
The CDC also finds that 2,756 of Americans aged 19 and under committed suicide in 2019 and 925 died of drowning.
Another 3,302 children died from traffic-related motor vehicle accidents in 2019.
What’s more, 3,371 children and teens in the U.S. lost their lives due to to gun violence in 2019, according to The State of America’s Children 2021 report.
Only bicycle accidents see fewer deaths with 79 occurring for those under age 20 in 2019, according to data from the U.S. Department of Transportation.
Fewer than 1 in 10 children infected with coronavirus suffer from ‘long Covid’
Very few children and teenagers infected with COVID-19 have long-term symptoms, a new study suggests.
Researchers from Harvard Medical School in Boston, Massachusetts, looked at more than 5,000 under-18s who contracted the virus.
They found that fewer than one in 10 children were battling so-called ‘long Covid’ three to five months after first testing positive.
Only 15 percent developed symptoms at any point during their infections, with most seeing symptoms disappear within 30 days.
The team says that the findings suggest long Covid is not as much of a concern among minors as it is among adults.
Long Covid appears in patients that have recovered from the virus and continue exhibiting symptoms for weeks, or potentially months or years, after clearing the infection.

The study found that 14.8% of children had symptoms between 14 and 30 days after testing positive, which fell to 7.2% having symptoms three months or later
There are a wide-array of symptoms that can appear, including continued loss of taste and smell, long-term fatigue and long-term sensory issues.
For the new study, published on pre-print server medRxiv.org, the team looked at 5,058 children and teenagers between ages five and 18.
All contracted Covid and were treated two unnamed New England health systems between March 2020 and April 2021.
The participants were followed up with monthly and up to five months after their first positive tests.
During the acute period of infection, between 14 and 30 days after first falling ill, 14.8 percent experienced symptoms.
This percentage fell to just 7.2 percent experiencing long-term symptoms more than three months later.
The most common symptoms were headache and anxiety, each with 2.4 percent of patients reporting these conditions.
Advertisement
Source link : https://www.dailymail.co.uk/health/article-10068863/Pfizer-asks-FDA-authorize-COVID-19-vaccine-kids-aged-5-11.html











