A panel of international experts representing the World Health Organization (WHO) has recommended high-risk COVID-19 patients receive Regeneron’s monoclonal antibody treatment to reduce their risk of severe disease.
Regeneron’s treatment – which the U.S. Food & Drug Administration (FDA) authorized in the U.S. in November – includes two antibodies, called casirivimab and imdevimab, that can boost patients’ immune systems.
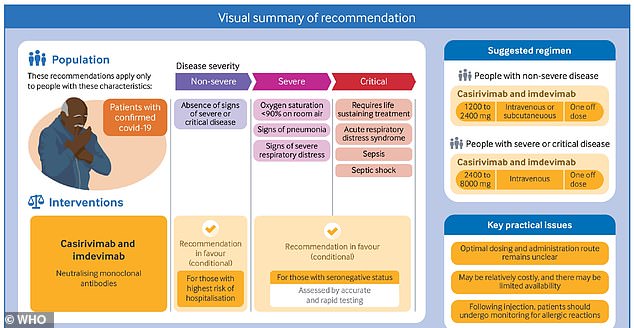
WHO recommends this treatment for two groups of patients: those who don’t have severe Covid but are at high risk of hospitalization, and those who have severe Covid and fail to test positive for coronavirus antibodies.
Still, the WHO panel acknowledges this treatment may be difficult to administer in low- and middle-income countries, due to its cost and equipment requirements.
It is even difficult to access in some U.S. states, due to new federal distribution protocols after just seven states comprised 70 percent of treatment orders.
The WHO has recommended Regeneron’s monoclonal antibody treatment, which boosts Covid patients’ immune systems. Pictured: A box and vial of Regeneron’s treatment at a treatment site in Orlando, Florida, August 2021
High-risk patients include those who don’t have severe Covid but are at high risk of hospitalization, and those who have severe Covid and fail to test positive for coronavirus antibodies
Monoclonal antibody treatments have been a popular strategy for treating Covid patients in the U.S., especially during the country’s recent surge.
In this treatment, a patient receives an infusion of synthetically-made antibodies – immune system proteins – that are designed specifically to fight the coronavirus.
If a patient is given this infusion soon after they become infected with the coronavirus, those synthetic antibodies can boost their immune system and ward off severe disease.
Data from the U.S. show that the treatment can help keep patients out of the hospital or, if they are hospitalized, it can help keep them out of intensive care units.
Former President Trump was treated with monoclonal antibodies when he contracted Covid in October 2020.
The FDA gave a monoclonal antibody treatment made by the drug company Regeneron in Emergency Use Authorization in November.
Now, a panel of experts representing the WHO has followed suit in recommending Regeneron’s treatment for patients globally.
Regeneron’s treatment includes two types of antibodies, called casirivimab and imdevimab. The antibody types work together to boost patients’ immune systems.
The WHO panel recommended this antibody treatment for two groups of patients.
The first group: patients who do not have a severe case of Covid, but may be at risk for developing severe symptoms due to their age, comorbidities, or other factors.
This recommendation is based on studies showing that Regeneron’s treatment reduces the risk of hospitalization for such patients, by making their symptoms less severe.
In addition, the WHO recommended that Regeneron’s treatment may be used for patients who have a severe case of Covid – but test negative for coronavirus antibodies, indicating that their immune system is not responding to the virus.
This second recommendation is based on data from a large trial, showing that the antibody treatment can boost the immune system response in critically ill patients.
For critically ill patients, Regeneron’s treatment can reduce the risk of death or requiring mechanical ventilation.
For other Covid patients, however, the WHO panel says that this treatment is less likely to have a significant impact.
Monoclonal antibody treatments are easier to access in the U.S. than in other countries. Pictured: A nurse prepares to administer Regeneron’s treatment at an urgent care center in Sarasota, Florida, September 2021
The WHO guidance is part of a living, often-updated guide to treating Covid, available at the journal BMJ.
In the guidance, the WHO experts acknowledged that it may be difficult for low- and middle-income countries to take advantage of Regeneron’s treatment.
The treatment costs $2,100 for one dose in the U.S. Costs may differ in other countries, via Regeneron’s partnership with pharmaceutical company Roche.
These nations may not have access to serological tests needed for prescribing the treatment, or to the intravenous equipment needed to administer the treatment.
In addition, the treatment may become less effective in future months if new variants emerge that are resistant to synthetic antibodies.
Regeneron’s treatment is currently difficult to access in some parts of the U.S., in fact, as the Department of Health and Human Services (HHS) has restricted its use due to recent supply concerns.
Last week, the HHS announced that it would control monoclonal antibody supplies because just seven states were making up 70 percent of orders for the treatment.
States such as Florida, which made monoclonal antibodies widely available through state-run clinics, are now facing high demand for the treatment.
The federal government is encouraging vaccines over monoclonal antibodies.
The cost of one monoclonal antibody dose is approximately 100 times the cost of one vaccine dose.
Source link : https://www.dailymail.co.uk/health/article-10022831/WHO-recommends-Regenerons-Covid-antibody-treatment-patients-high-risk-hospitalized.html











