Drug manufacturer Eli Lilly and Co revealed its COVID-19 drug reduced the risk of death in patients on mechanical ventilation by close to half.
The Indianapolis-based company released data on Tuesday from its late-stage clinical trial of the arthritis drug, baricitinib, which was developed in partnership with pharmaceutical firm Incyte.
Patients put on ventilators who received baricitinib plus standard of care were 46 percent less likely to die compared with those given a placebo.
What’s more, the researchers found one death was prevented for every six baricitinib-treated patients on mechanical ventilation compared to a placebo.
A new study looked at 101 adult COVID-19 patients who were hospitalized either with mechanical ventilation or ECMO machines in which half were treated with the arthritis drug, baricitinib (pictured) plus standard of care and the other half were given a placebo
Researchers found patients who received baricitinib were 46% less likely to die four weeks later compared to those in the placebo group. Pictured: Critical Care Nurse Emily Bouche takes care of COVID-19 patient Hannah Church, 25, at Johnston Memorial Hospital in Abingdon, Virginia, June 2021
‘As additional data…become available, it is increasingly evident that treatment with baricitinib may help prevent death in some of the most critically ill COVID-19 patients,’ said Dr E Wesle Ely, a professor of medicine at Vanderbilt University and co-principal investigator of the study, in a press release.
‘Baricitinib represents an important treatment option for this vulnerable group of patients in this constantly evolving pandemic.’
For the study, the team looked at 101 adult patients who were hospitalized either with mechanical ventilation or Extracorporeal Membrane Oxygenation (ECMO) machines.
The machine, typically used for those with heart and lung issues, pumps and oxygenates a patient’s blood outside the body, which allows the heart and lungs to rest.
A total of 39.2 percent of patients who received baricitinib plus standard of care, or 20 out of 51, died compare to 58 percent, or 29 of 50 patients, given a placebo by day 28.
By day 60, the percentages had risen to 45.1 percent in the treatment arm compared to 62.0 percent for the control group.
Researchers concluded patients who received baricitinib were 46 percent less likely to die four weeks later compared to those in the placebo group.
‘In the interest of public health and safety, it remains a priority to provide healthcare professionals with as much information as possible about treatment options that may help improve outcomes for patients with severe disease,’ said Dr Ilya Yuffa, senior vice president and president of Lilly Bio-Medicines.
‘These new data add to the growing body of evidence demonstrating the important role baricitinib has and may continue to play for certain hospitalized patients with COVID-19.’
The U.S. Food and Drug Administration (FDA) first approved baricitinib in combination with Gilead Sciences’ remdesivir, to treat COVID-19 patients.
Last month, the agency expanded the drug’s authorization for lone use or with remdesivir.
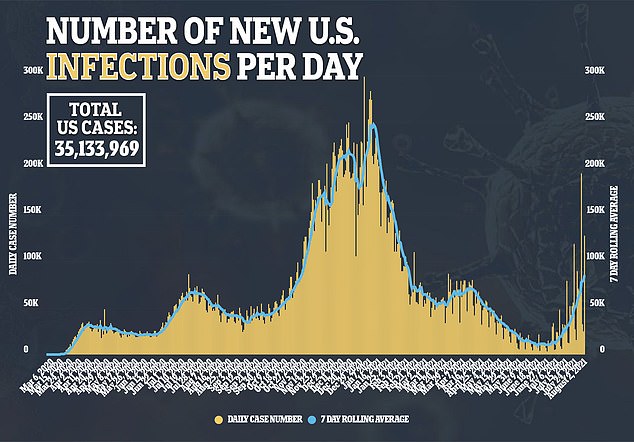
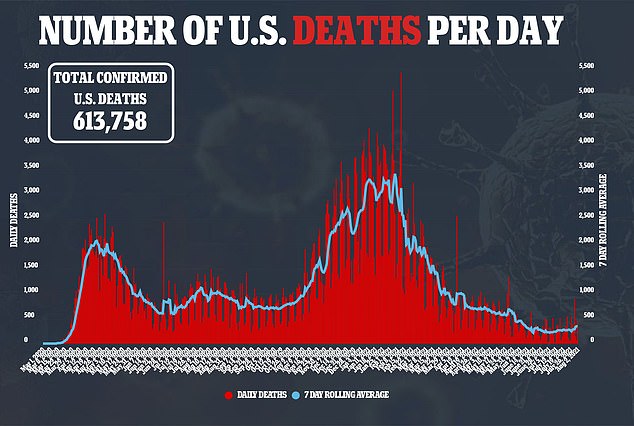
Baricitinib is not the only arthritis drug approved to treated COVID-19 with tocilizumab, sold under the brand name Actemra, also approved.
These medications belonged to a class of drugs called interleukin-6 (IL-6) inhibitors that could help mitigate a dangerous overreaction to the virus by the body’s immune system called a cytokine storm.
These so-called storms occur when the body doesn’t just fight off the virus but also attacks its own cells and tissues, and were a notorious cause of death for COVID patients during the initial months of the outbreak .
In cases of COVID-19, the disease caused by the virus, cytokine storms can trigger respiratory distress, which can lead to multi-system organ failure and death.
Lilly said the new data from the study will be shared with regulatory authorities in the U.S., European Union and other areas.
Source link : https://www.dailymail.co.uk/health/article-9855843/Lillys-COVID-19-drug-reduces-death-risk-patients-mechanical-ventilation.html











