People who received Johnson & Johnson’s COVID-19 vaccine have higher antibody levels if their booster shot is from Pfizer-BioNTech or Moderna, a new report suggests.
The National Institutes of Health (NIH) has allegedly collected data showing that mixing-and-matching is more protective than getting two doses from the New Brunswick, New Jersey-based company, a person who has seen the data told Axios.
NIH officials plan to present the findings during a meeting of the U.S. Food and Drug Administration’s (FDA) vaccine advisory committee on Friday.
It comes as J&J has asked the FDA to approve a shot of its own single-dose vaccine as the booster dose.
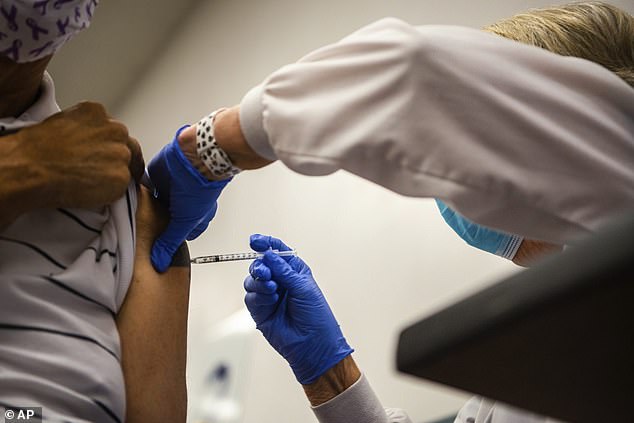
The FDA’s vaccine advisory committee is meeting on Friday to discuss the need for booster shots of Johnson & Johnson’s COVID-19 vaccine. Pictured: Diana Serlo, a retired registered nurse from Excela, administers a COVID-19 vaccine booster shot in North Huntington, Pennsylvania, September 2021
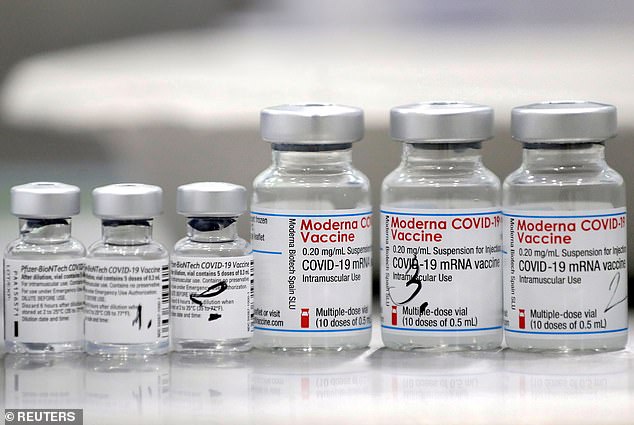
The NIH is planning to present data showing J&J recipients have higher antibody levels if they get a booster shot from Pfizer or Moderna. Pictured: Vials of Pfizer-BioNTech and Moderna COVID-19 vaccines, February 2021
According to Axios, the NIH data is presumed to show ‘a significantly stronger neutralizing antibody response’ from a Pfizer or Moderna booster than from a second dose of J&J.
It’s currently unclear if the recipients received a full or half dose of the booster shot and how long after their first shot they received a boosters.
However, there were limitations to the NIH data, according to the report.
Neutralizing antibodies are only type of immune response, preventing the the virus from entering cells and replicating, and the report said it was unclear how long the response will last.
The NIH, FDA and J&J did not immediately respond to DailyMail.com’s requests for comment.
As of Wednesday, about 15.2 million Americans have received J&J’s vaccine, which is administered as a single dose,
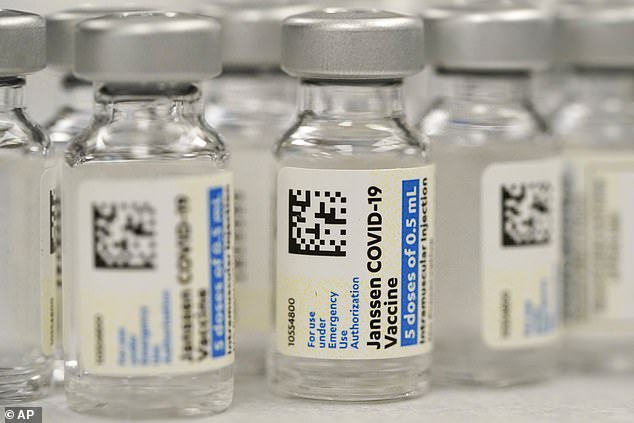
J&J has asked the FDA to approve a shot of its own single-dose vaccine as the booster dose. Pictured: Vials of J&J COVID-19 vaccine at a pharmacy in Denver, October 2021
Last month, the firm published the results of a study looking at an additional second shot of its vaccine.
The Phase III two-dose trial of up to 30,000 participants looking at the effectiveness of a second dose given 56 days after the first in adults 18 and older.
Results showed that a booster shot was 94 percent effective against symptomatic COVID-19 in the U.S. and 100 percent effective against critical illness at least 14 days post-vaccination.
This compares to 70 percent protection seen with a single dose.
There was only one case of COVID-19 in the vaccine group and 14 in the placebo group.
J&J said that a booster given two months after the first dose increased antibody levels between four-fold and six-fold.
When given six months after the first dose, antibody levels shot up nine-fold after one week and 12-fold after four weeks.
These increases were seen regardless of age.
Side effects with two doses were comparable to those seen in studies with the single-dose vaccine.
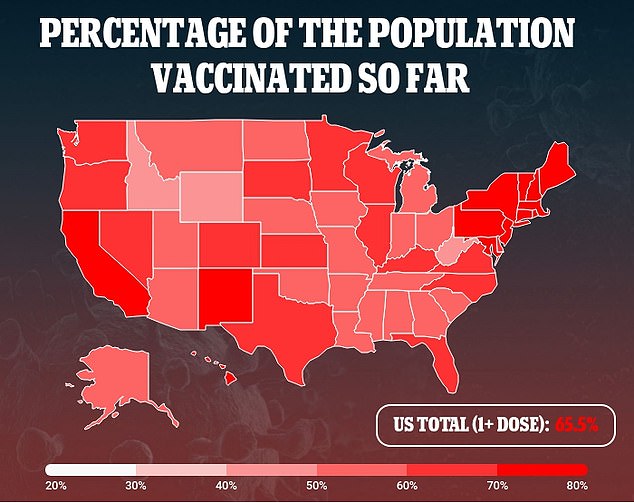
‘A single-shot COVID-19 vaccine that is easy to use, distribute and administer, and that provides strong and long-lasting protection is crucial to vaccinating the global population,’ Dr Paul Stoffels, J&J’s chief scientific officer, said in a statement at the time.
‘At the same time, we now have generated evidence that a booster shot further increases protection against COVID-19 and is expected to extend the duration of protection significantly.’
This week, the FDA advisory committee will also meet this week to discuss the need for an additional dose of Moderna’s vaccine.
Scientists at the FDA have said Moderna had not met all of the agency’s criteria to support use of booster doses of its COVID-19 vaccine, possibly because the efficacy of the shot’s first two doses has remained strong.
Source link : https://www.dailymail.co.uk/health/article-10086657/Data-suggests-mRNA-booster-dose-generates-stronger-antibody-response-J-J-shot-Axios.html











