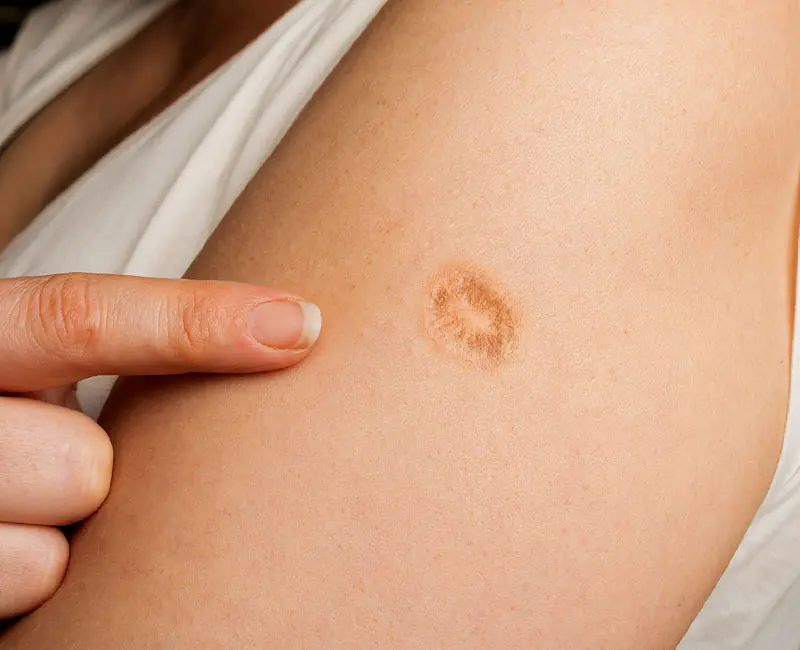
We got it when we were children; nobody asked, nobody explained. Today, few know the truth behind this mark that so many of us have
We got it when we were children; nobody asked, nobody explained. Today, few know the truth behind this mark that so many of us have

Mix Soap with Sugar: A Simple Household Trick with Surprisingly Useful Benefits
This Simple Kitchen Trick May Help Get Rid of Cockroaches and Rats

4 Ways to Reduce Age Spots with Baking Soda for Brighter, More Even Skin
Dark Spots on Your Face? Try These Simple Baking Soda Tricks
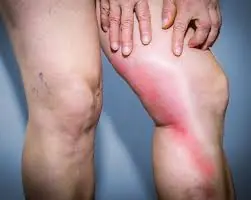
Heart Health Alert: 7 Silent Warning Signs You Should Never Ignore
Heart Health Alert: 7 Silent Warning Signs You Should Never Ignore
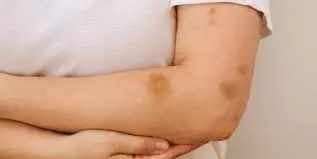
If You’re Getting Bruises Without Injury, Here’s What Your Body Might Be Telling You
If You’re Getting Bruises Without Injury, Here’s What Your Body Might Be Telling You

Eating Chicken Eggs Can Be Harmful for These 5 Groups of People — Here’s Why
5 Types of People Who Should Limit Eating Eggs — And the Reasons Why
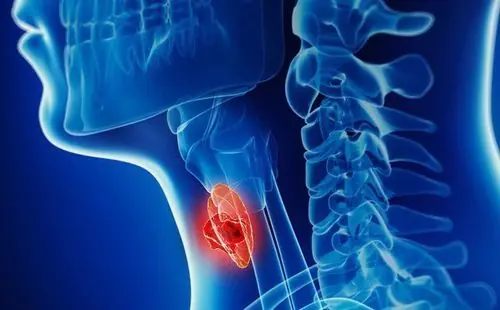
Sticky Throat? Here Are the Real Reasons Your Throat Feels Full of Mucus
Doctors Explain Why Your Throat Feels Mucusy and Sticky

How to Safely Remove the Wax Coating on Apples Before Eating
The Best Methods to Get Rid of the Wax Coating on Apples
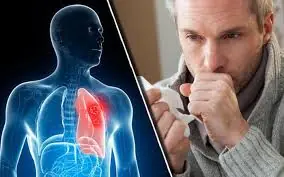
Don’t Ignore These 4 Morning Symptoms—Doctors Say They Could Signal Lung Cancer Progression
Waking Up With These 4 Symptoms? Doctors Say It Could Be a Sign of Advancing Lung Cancer

Three Members of the Same Family Diagnosed with Thy.roid Can.cer — Doctors Found the Culprit Right on the Dining Table
This common food found in many homes may carry hidden health risks if eaten too often.

7 Subtle Symptoms Many Women Ignore — But They Could Be Warning Signs of a Stroke
7 Subtle Symptoms Many Women Ignore — But They Could Be Warning Signs of a Stroke
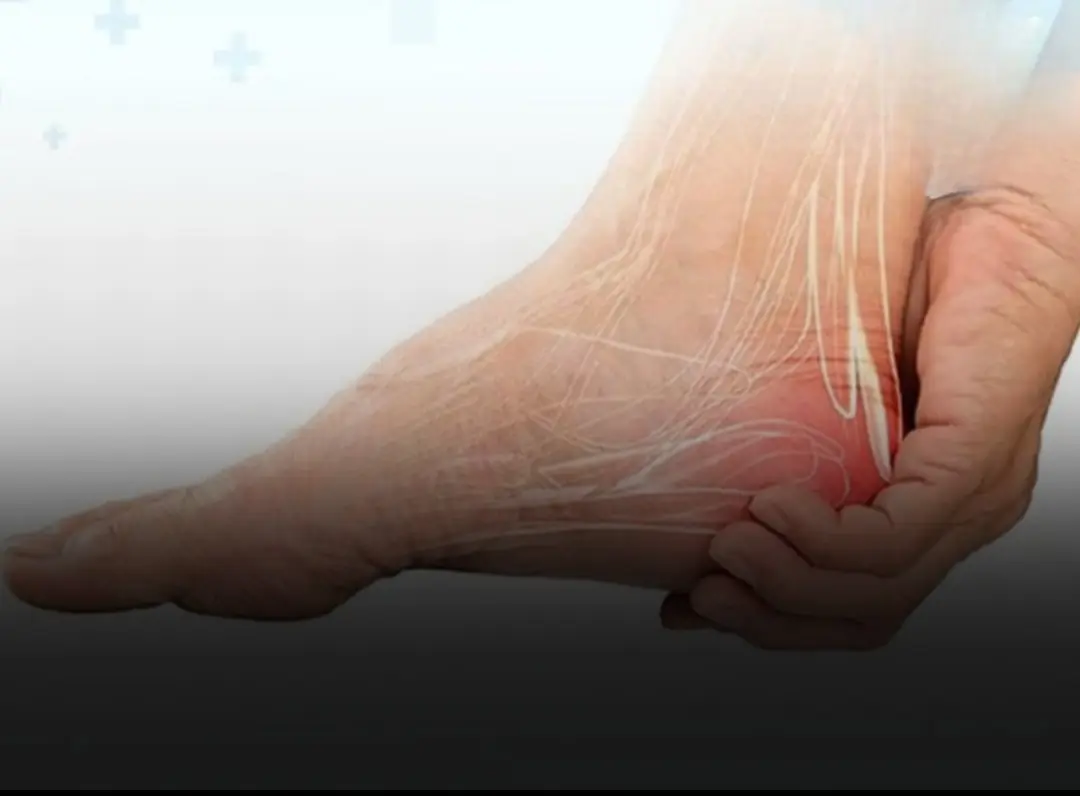
If Your Heel Hurts When You Wake Up or After Standing for a Long Time
If Your Heel Hurts When You Wake Up or After Standing for a Long Time

How to make your tiles shine at home using simple household methods
Make Your Tiles Look Brand New with These Free Home Cleaning Tips
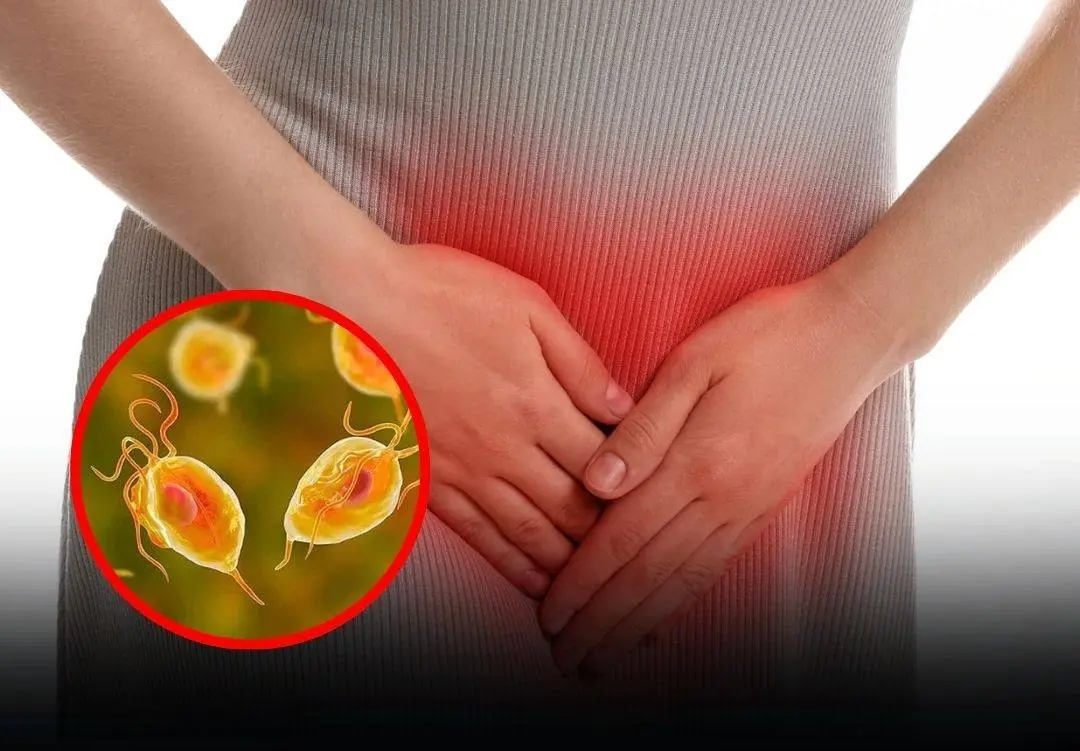
In everyday routines, women tend to be highly mindful of personal hygiene, particularly intimate care
In everyday routines, women tend to be highly mindful of personal hygiene, particularly intimate care
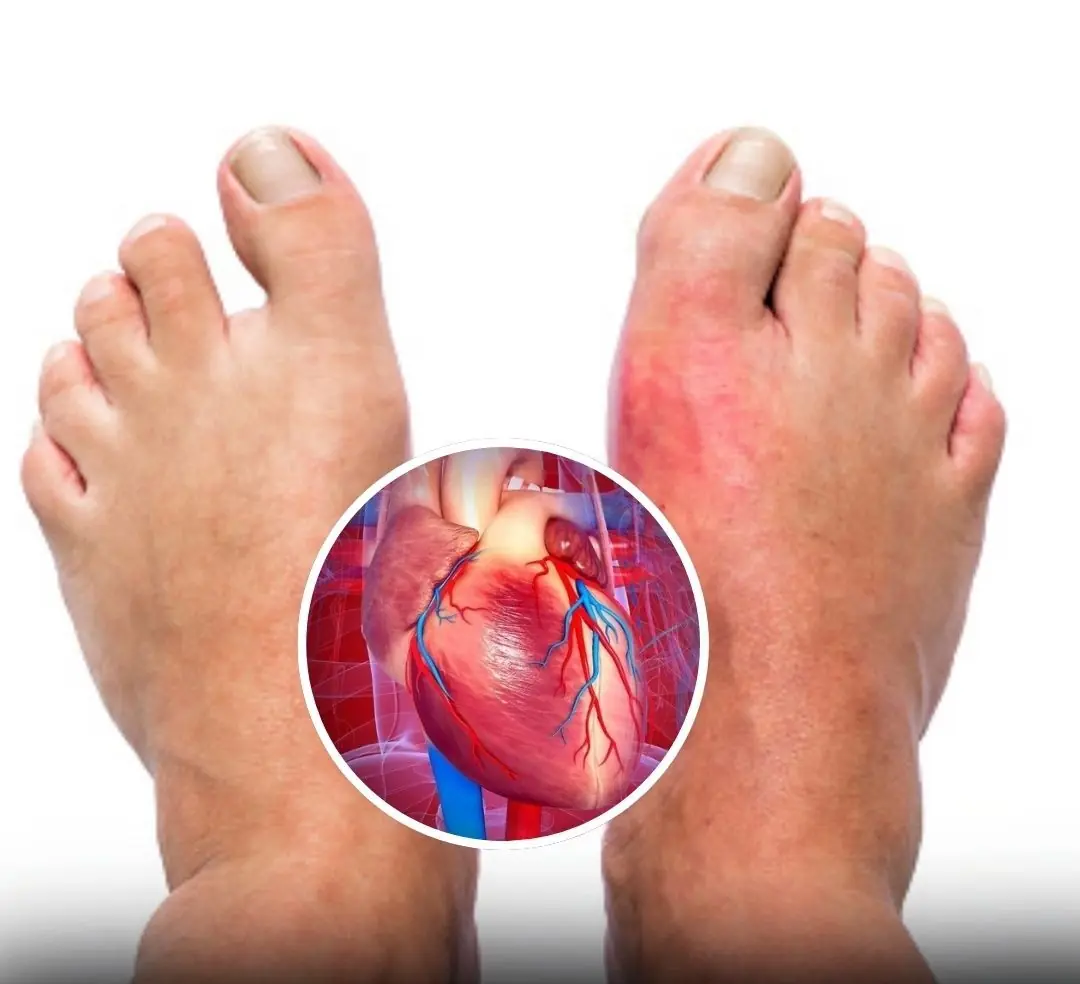
One Month Before a Heart Attack, Your Body May Warn You With These 7 Signs
One Month Before a Heart Attack, Your Body May Warn You With These 7 Signs
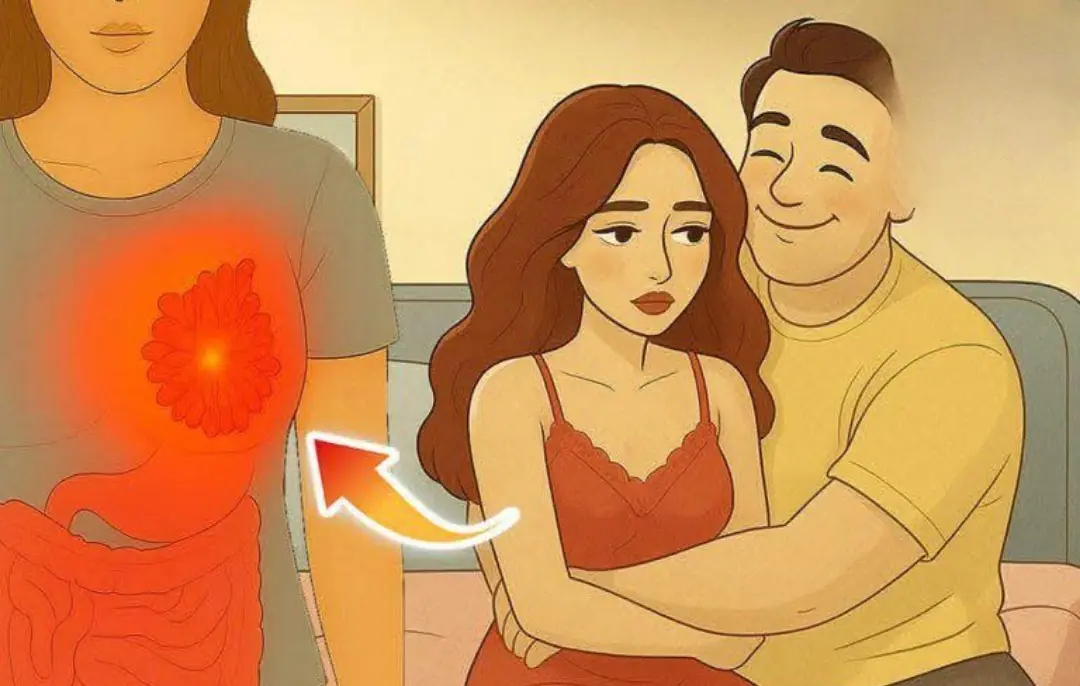
Wives’ bre.ast can.cer risk linked to husbands’ unhealthy habits
Wives’ bre.ast can.cer risk linked to husbands’ unhealthy habits

Stretch Your Ring Finger With Your Thumb for a Few Seconds — You’ll Love the Reason
Stretch Your Ring Finger With Your Thumb for a Few Seconds — You’ll Love the Reason
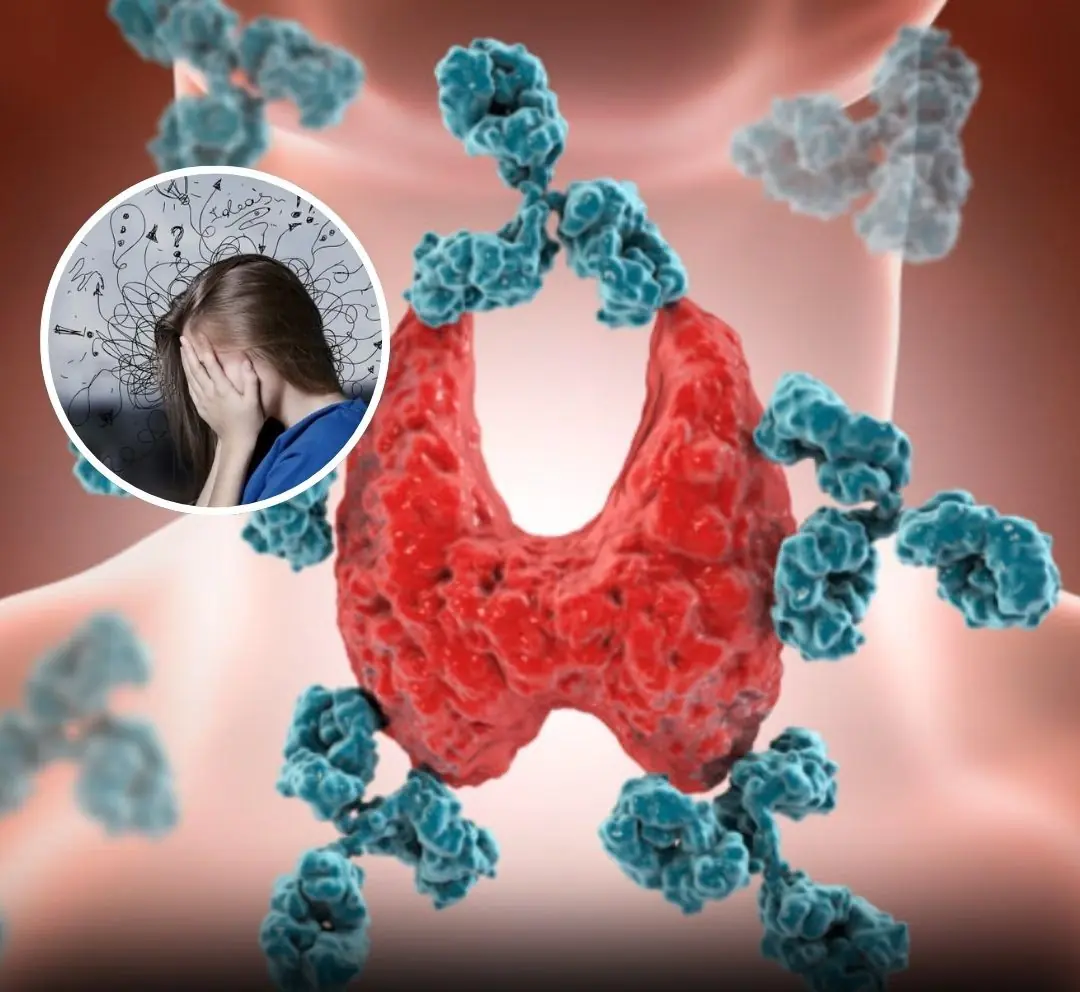
Learn how people-pleasing affects your health and triggers autoimmune disorders—details in the comments below!.....
Learn how people-pleasing affects your health and triggers autoimmune disorders—details in the comments below!.....

Doctors Explain What Eating Boiled Eggs in the Morning May Do to Your Body
Boiled eggs in the morning may offer surprising health benefits.

9 Silent Warning Signs of a Brain Blo:od Clot
9 Silent Warning Signs of a Brain Blo:od Clot



















