
These individuals shouldn't eat even a bite
Eggplant is delicious, but not everyone should eat it. Here are the people who should absolutely avoid this vegetable:
1. People with Anemia or Iron Deficiency
Eggplant skin contains anthocyanin. This substance "captures" iron ions present in other foods and in the body, hindering the body's absorption of iron. Additionally, it also affects the absorption of zinc and copper ions. Therefore, people with anemia or iron deficiency should avoid eating eggplant and instead, consume iron-rich foods like red meat and animal liver.

2. People with Stomach Issues
As a food with a cooling nature, eating too much eggplant can cause stomach discomfort and lead to diarrhea. Therefore, individuals with stomach problems should limit their intake of this vegetable.
3. People with Poor Digestive Function
Even though they might not experience stomach aches or indigestion like children, individuals with poor digestive function can still feel discomfort when eating eggplant due to its tough and hard skin. If they want to eat eggplant, this group should peel the skin to reduce the burden on their stomach.
4. People with Kidney Disease
Eggplant contains a large amount of oxalate – a type of acid found in plants. Consuming too much can lead to kidney stones. Hence, people with kidney disease should avoid eating eggplant.
News in the same category

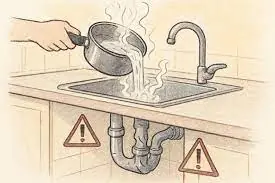
Stop pouring hot water down the sink — here’s why!

The Special Purpose of the Two Small Holes on Flat Plug Prongs That Many People Don’t Know

Here's how to choose delicious, sweet mangosteens – all 10 of them are perfect

A 111-year-old man eats these two foods every day—and they’re incredibly cheap at local markets

How to Quickly Eliminate Bed Bugs, Cockroaches, Fruit Flies, and Other Insects from Your Home

Mosquitoes are terrified of this leaf: Place a handful in your home and not a single one will dare to buzz around

Why do people put garlic at the head of the bed before going to sleep? The reason may surprise you.

Snakes are af.raid of these 5 plants - Plant them around your house to repel snakes and protect your family

90% of women don’t know this trick: Add this one thing to the pan and you can fry “everything” without worrying about oil splattering!

When Buying Bananas, Just Say These 3 Words — Sellers Will Think You’re an Expert and Won’t Dare to Cheat You

Stop Storing Ginger in the Fridge! Here’s How to Keep It Fresh for Up to 6 Months

Thought It Was Just Kitchen Waste, Lemon Peels Turn Out to Be a “Hidden Treasure” With 5 Little-Known Uses

Stop washing clothes the old way! Don’t just add detergent—try this quick hack and your clothes will come out like new.

Expert reveals 'military sleep method' that helps you fall asleep in just two minutes

Don’t Fry Fish with Just Oil: Add These 3 Ingredients for Golden, Crispy Fish with No Oil Splatter

Warning for Anyone Using an Air Fryer: There’s One Essential Part You Must Clean—but It’s Often Overlooked

Fridge leaking water: Don't rush to call a technician, just do this to keep your fridge running smoothly without spending money

7 Power-Hungry Home Appliances: Unplugging Them Can Save Electricity—but Also Shorten Their Lifespan

Boil eggshells and say goodbye to the …
News Post

6 foods that silently drain calcium from your body
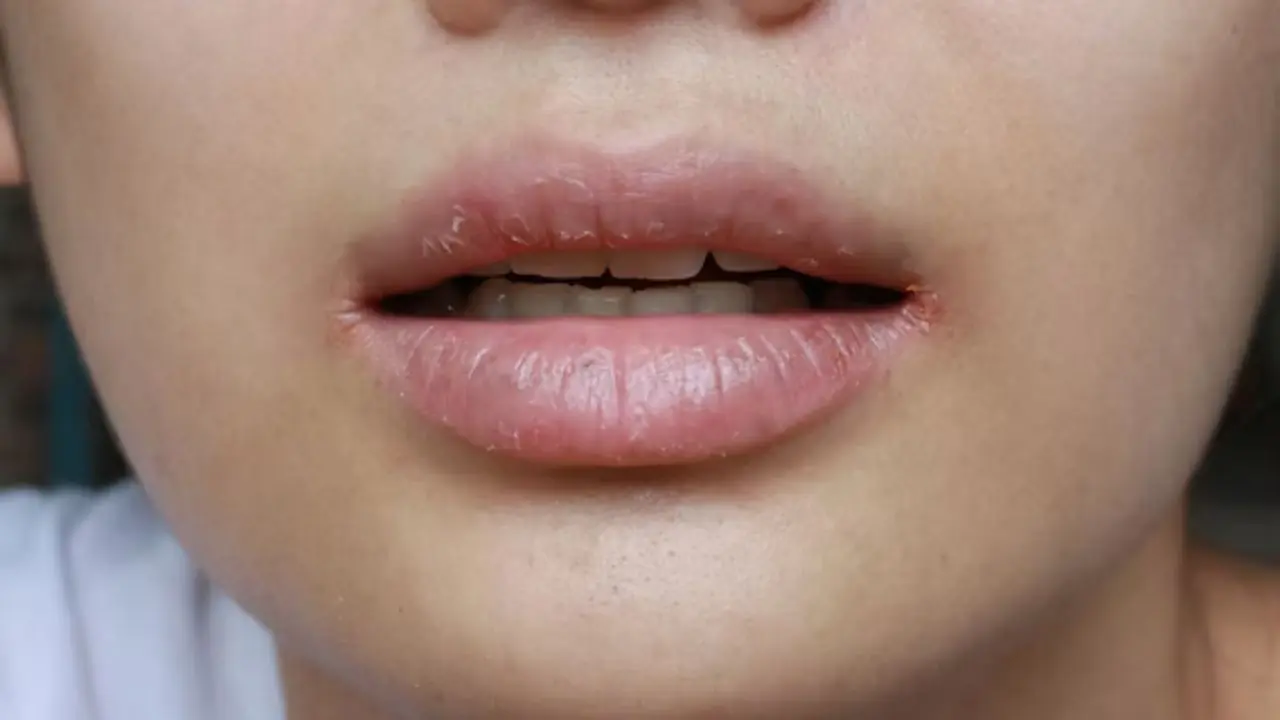
People whose mouths feel dry at night need to know these 8 possible reasons
Não ignore estes 7 sintomas matinais - eles podem estar ligados ao câncer

Cube Steak with Onion Gravy
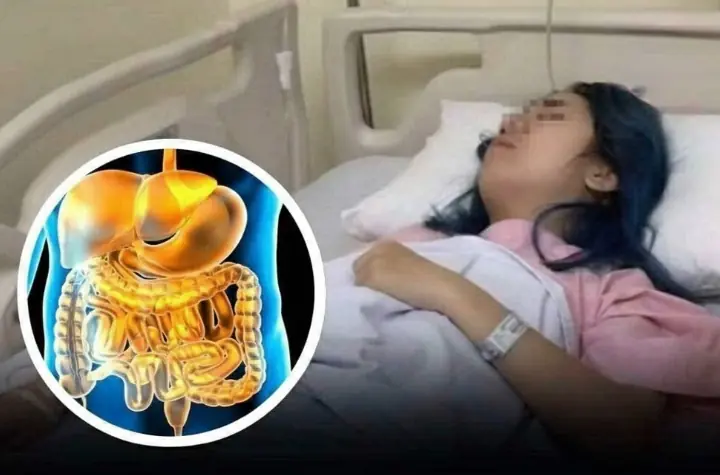
Eat 4 foods on an empty stomach in the morning to help clean the intestines, improve digestion and prevent can.cer

What does having cold hands and feet indicate?
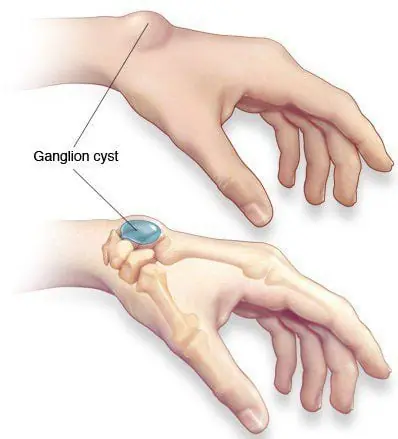
An unusual lump or bump appears on the wrist: Don't ignore it, as it could be a warning sign of a serious illness
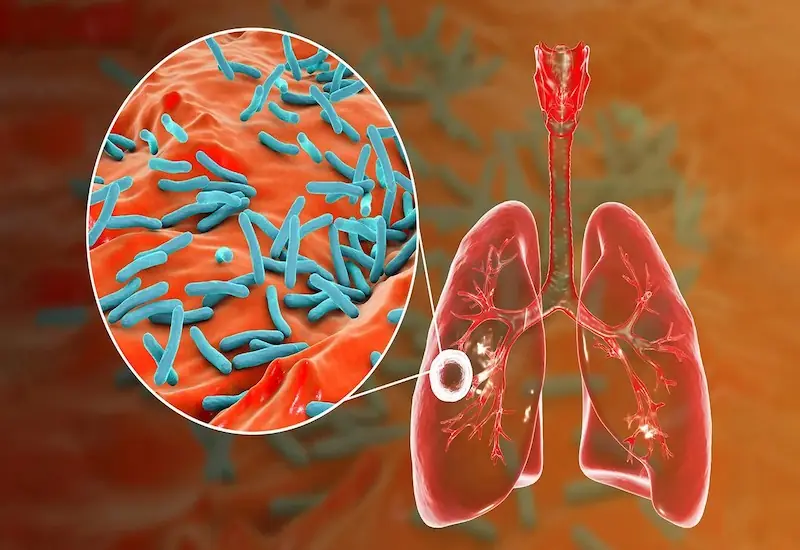
A healthy 22-year-old man suddenly discovered da.nger.ous tu.bercu.losis from a sign that many people ignore

Chocolate Lava Cake
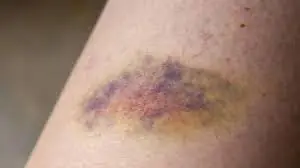
Bruises on the body - a warning that you may have a problem

Spicy Beef Noodle Bowl with Chili Oil

Yogurt and Fruit Parfaits
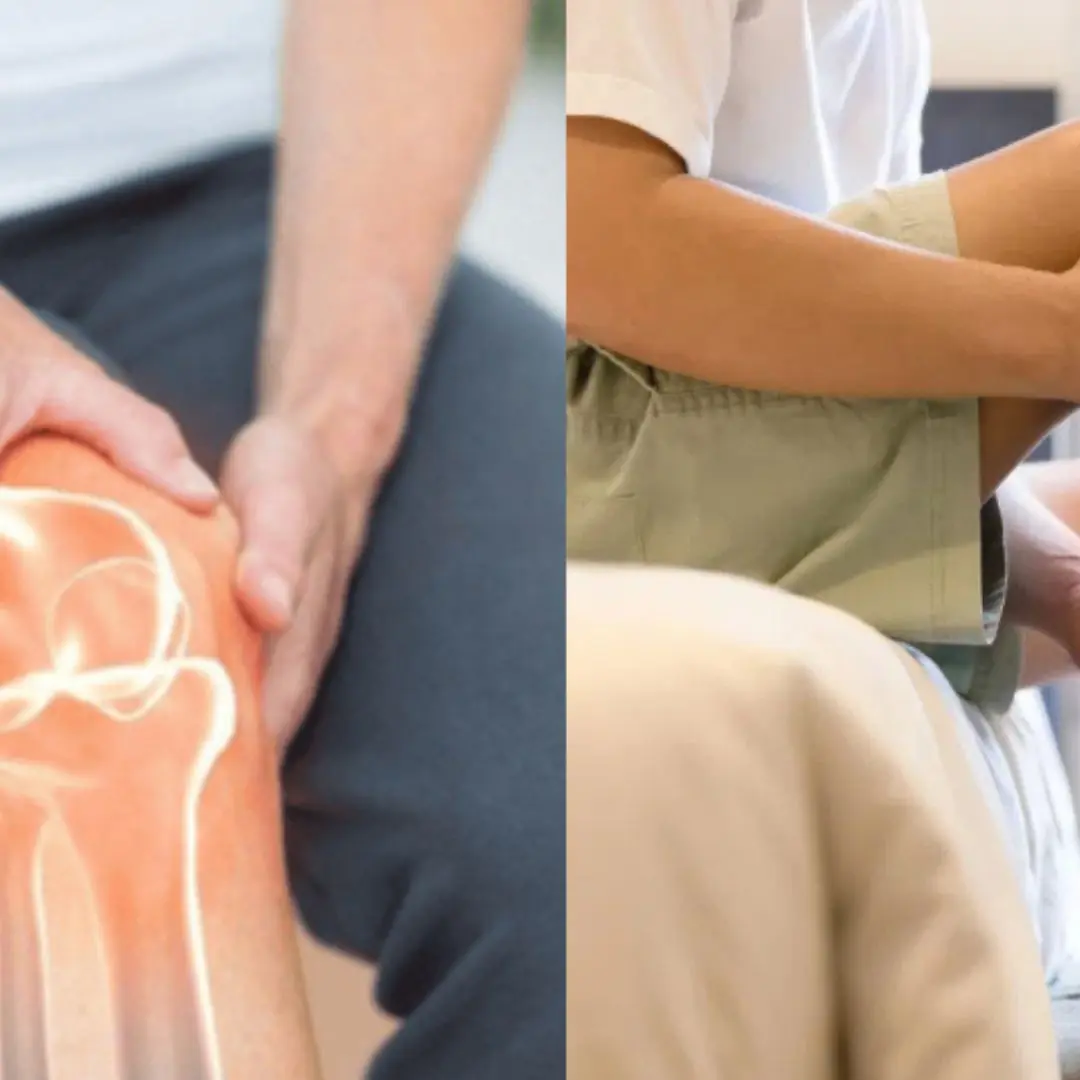
People with calcium deficiency often experience these 7 signs. Check now to see if you have them

The amazing hidden power of raspberry leaf you may not know

Doctors Warn: This Common Way of Eating Boiled Eggs Can Clog Your Arteries

Luxurious Lobster Bites in Aromatic Garlic Butter Sauce
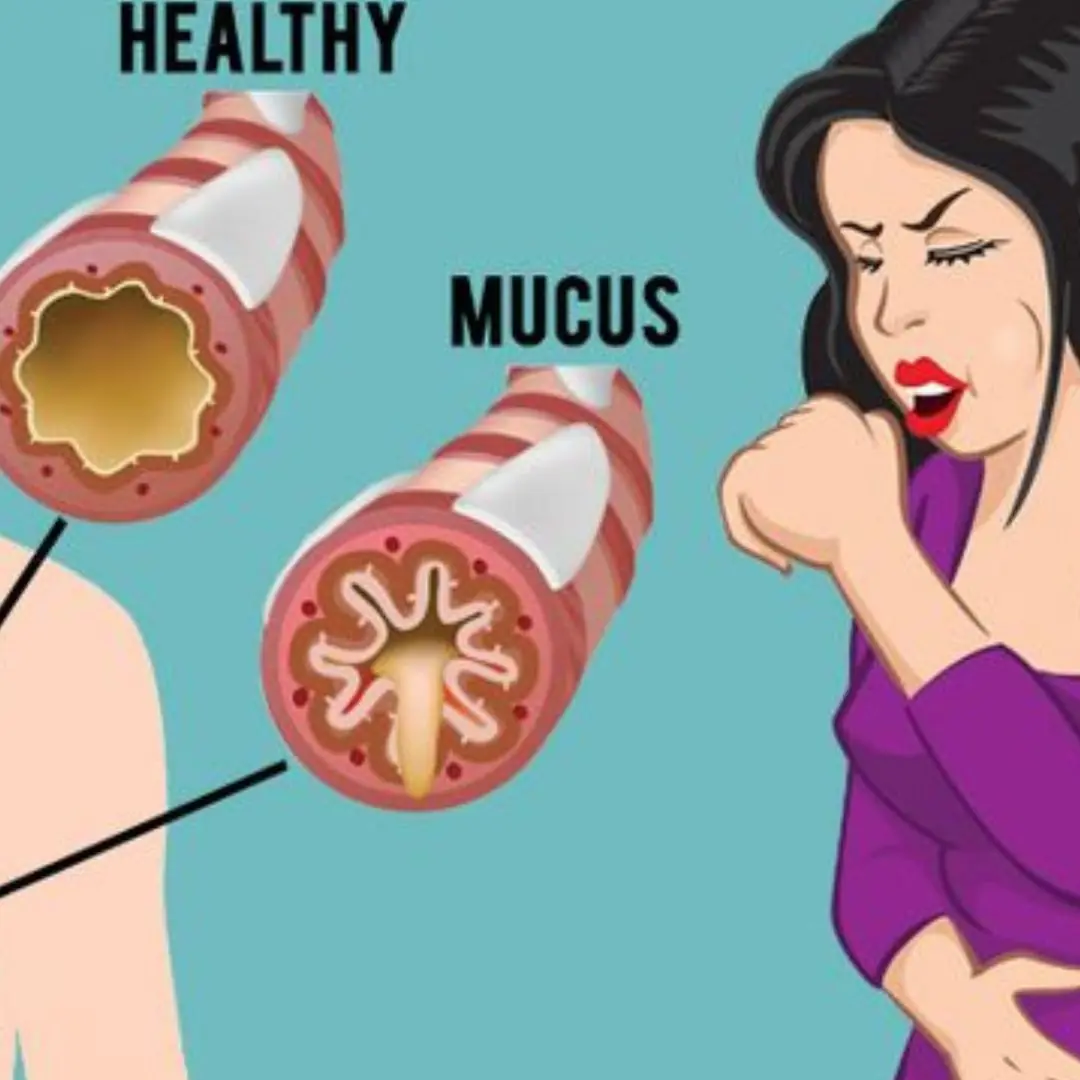
Effective Ways to Clear Phlegm and Mucus From Your Chest and Throat
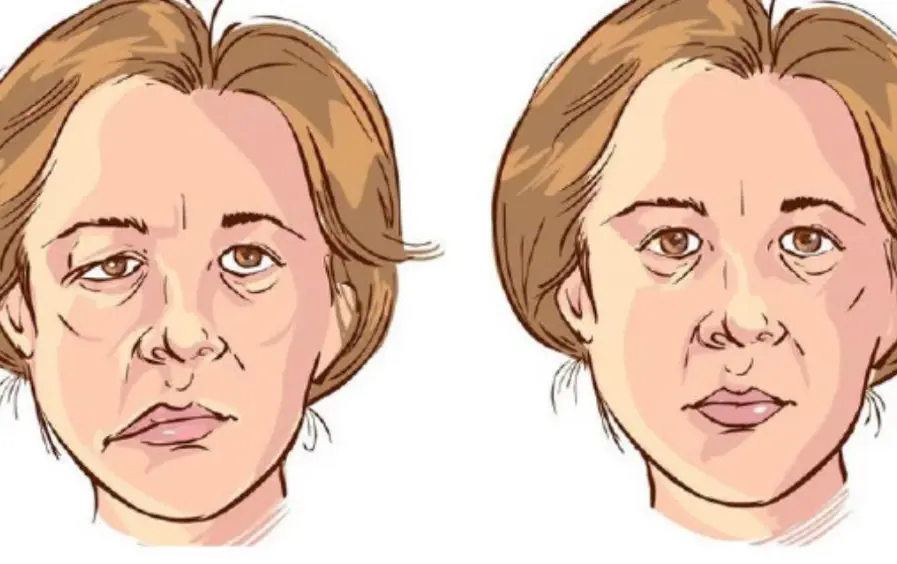
Early Warning Signs of a Str.oke That May Appear 90 Days in Advance

Teeth grinding while sleeping - normal action or sign of disease
