
Many brothers still do not understand why

The answer is: When your crush passes by you, you're fine, but when your crush says hello, you cry tears of joy.
News in the same category


If you know what this is, you had a very wild chilhood
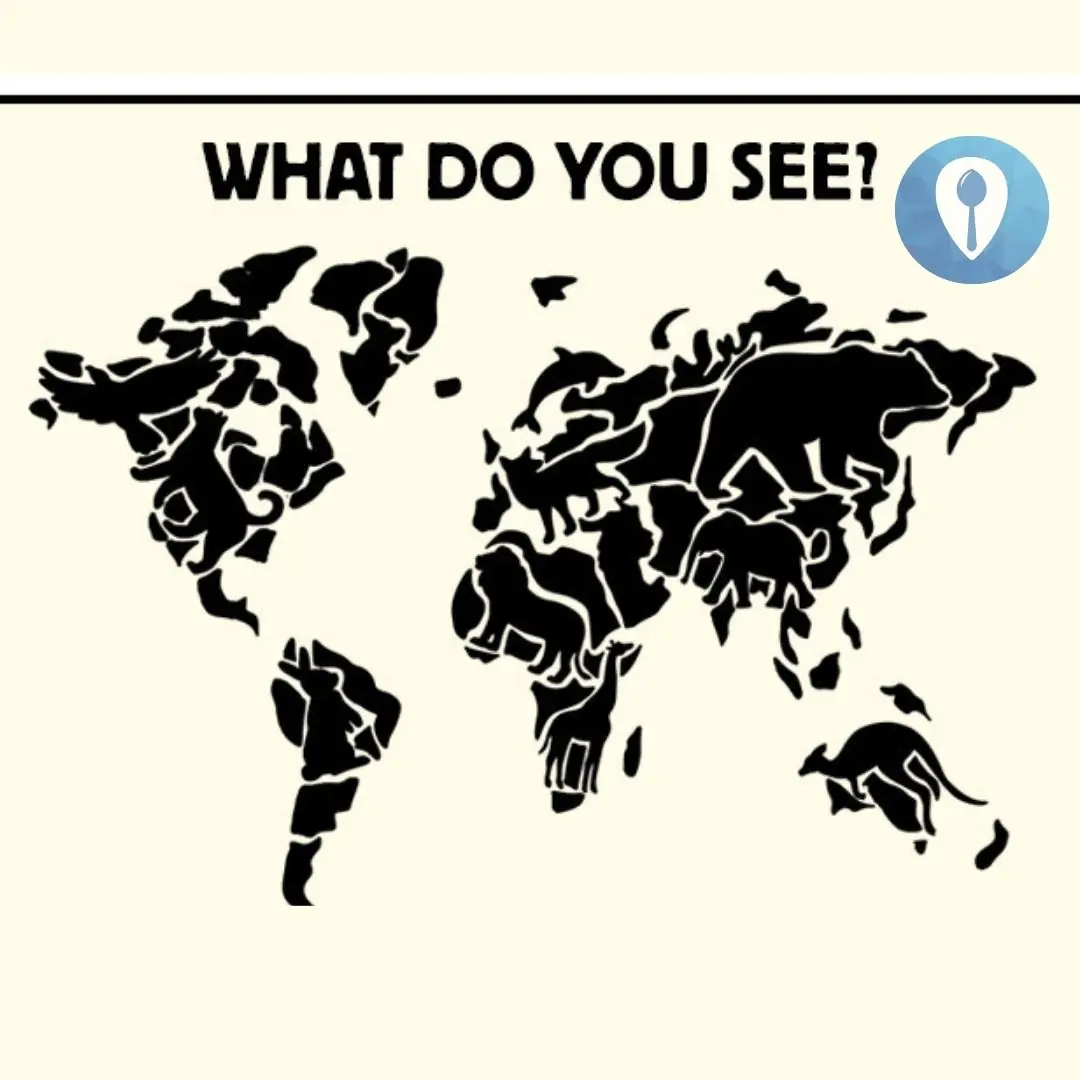
What Animal You See First Will Reveal Your Anger Trigger

This key only opens one of the five cars — can you guess which one?

People Are Desperately Searching for It — But Only a Few Know Where to Find It!

The ring you pick will reveal your truest trait
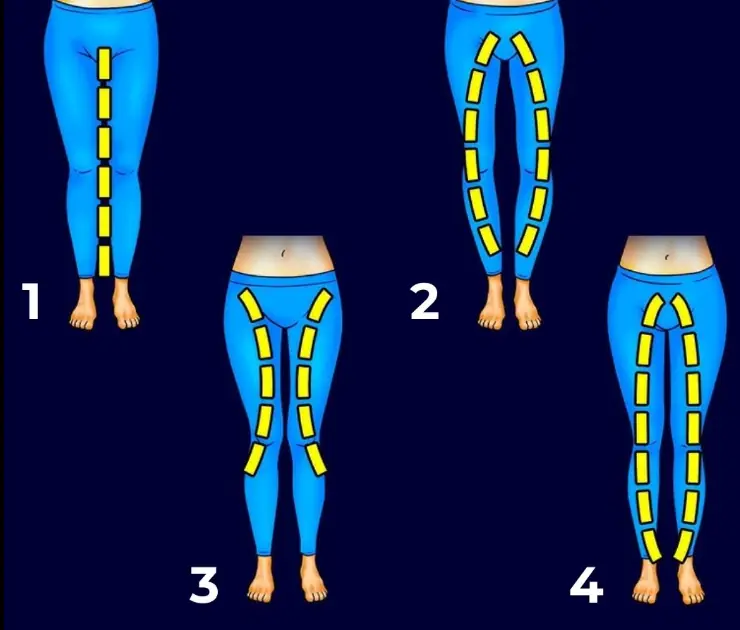
What your leg shape might reveal about your personality?

Most of you can't find the frog hiding in the picture, what about you?

Son took his mother to a nursing home and only visited her from time to time
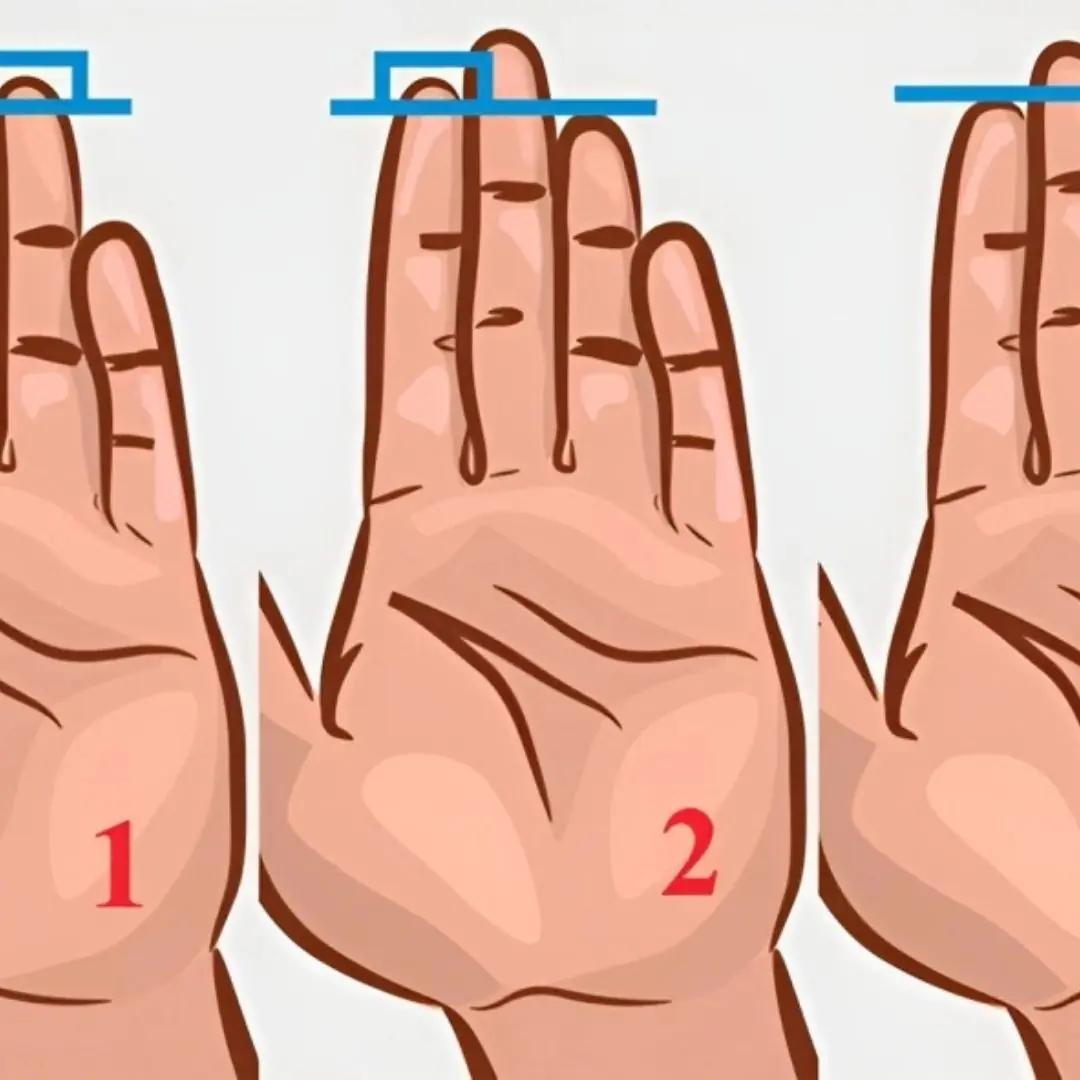
Ring Finger Length Reveals Interesting Personality Traits
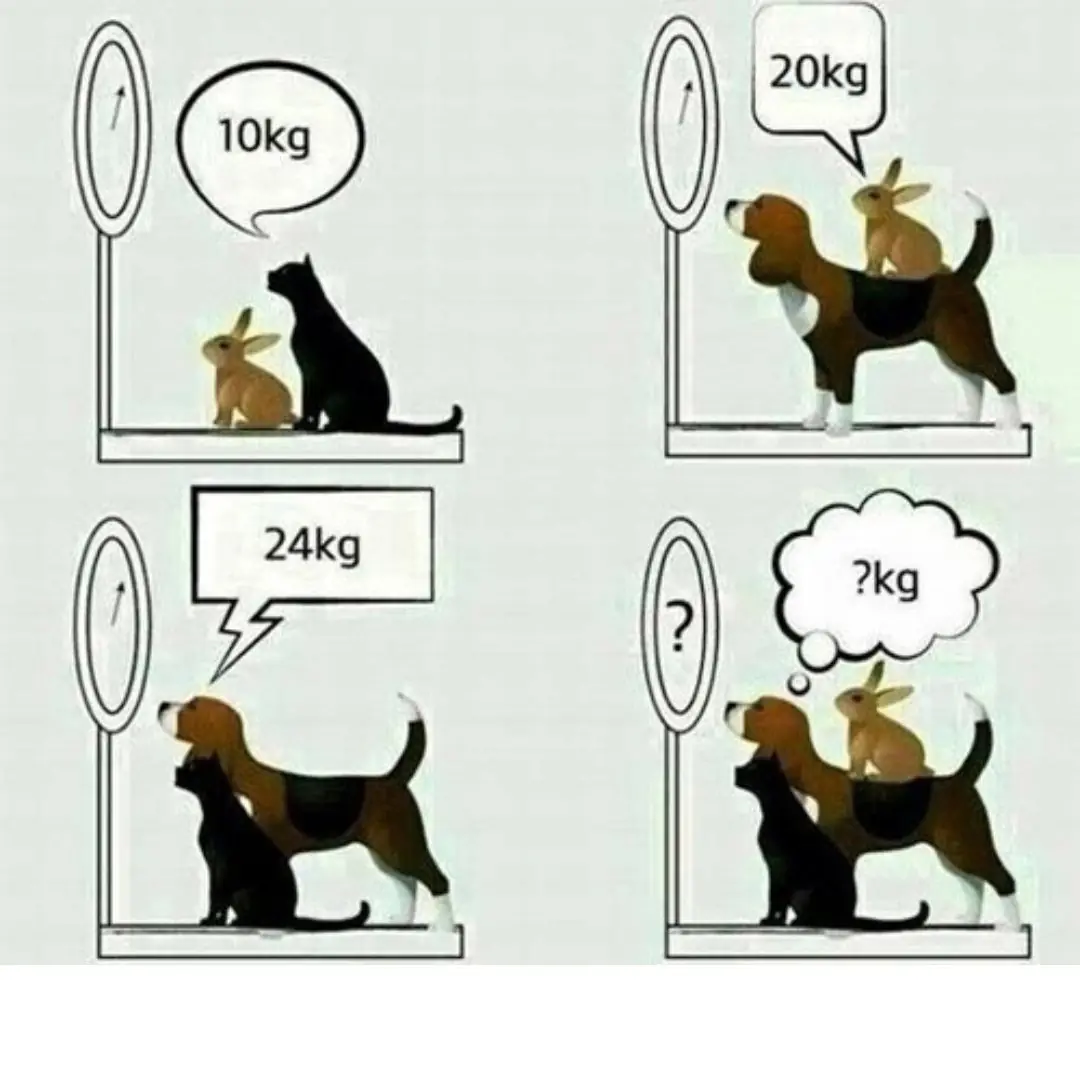
What is the total weig.ht of the 3 animals?

You only have 5 seconds! Where is the frog hiding, do you see?

Where is the 4th cat?
Squint your eyes and guess what animals are hiding behind these illusions

Only 1% of people guess correctly this fruit associated with childhood – are you one of them?

Can you spot the two ch.il.dren hidden in the picture?
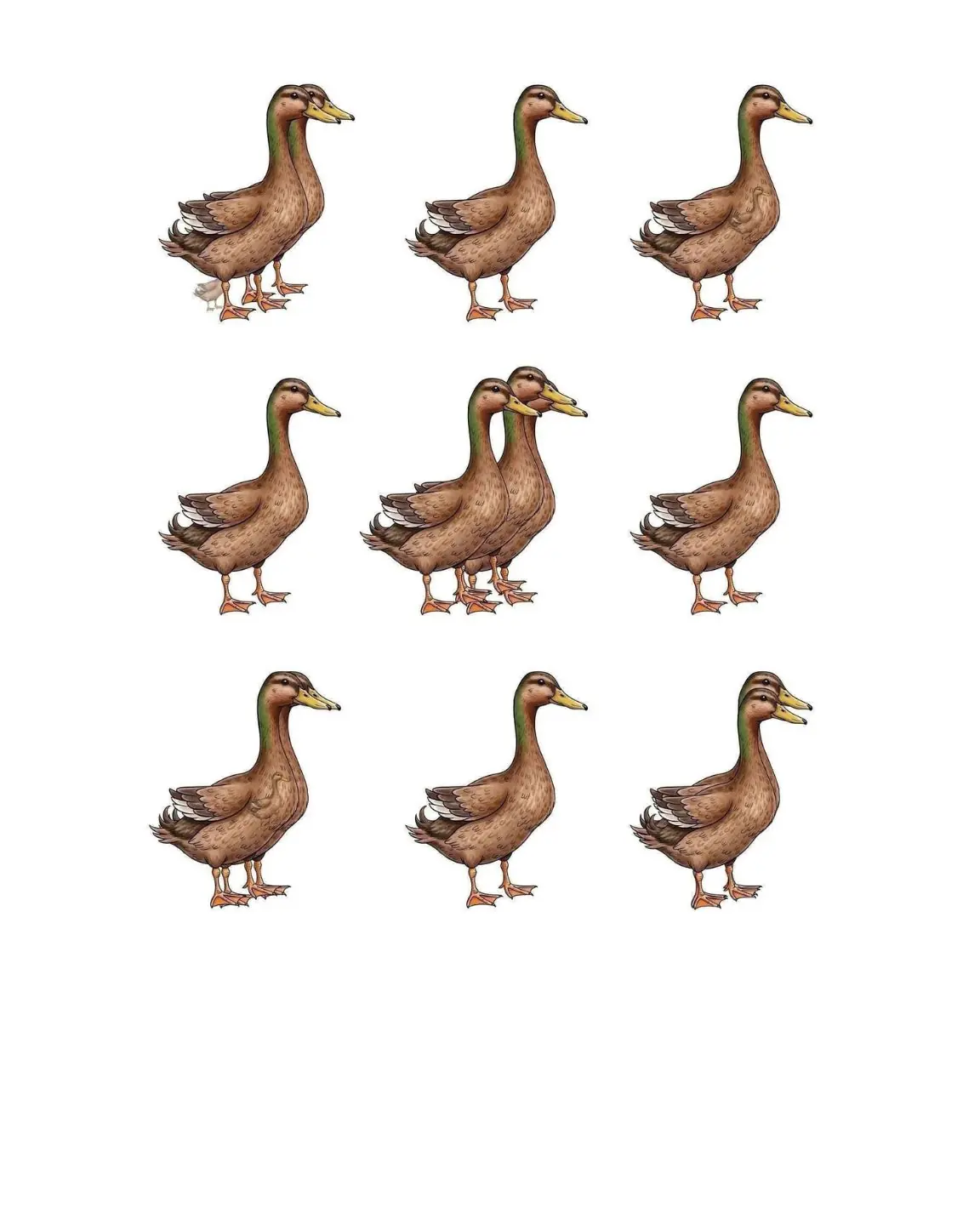
I spotted 10 ducks—did you get the same number?
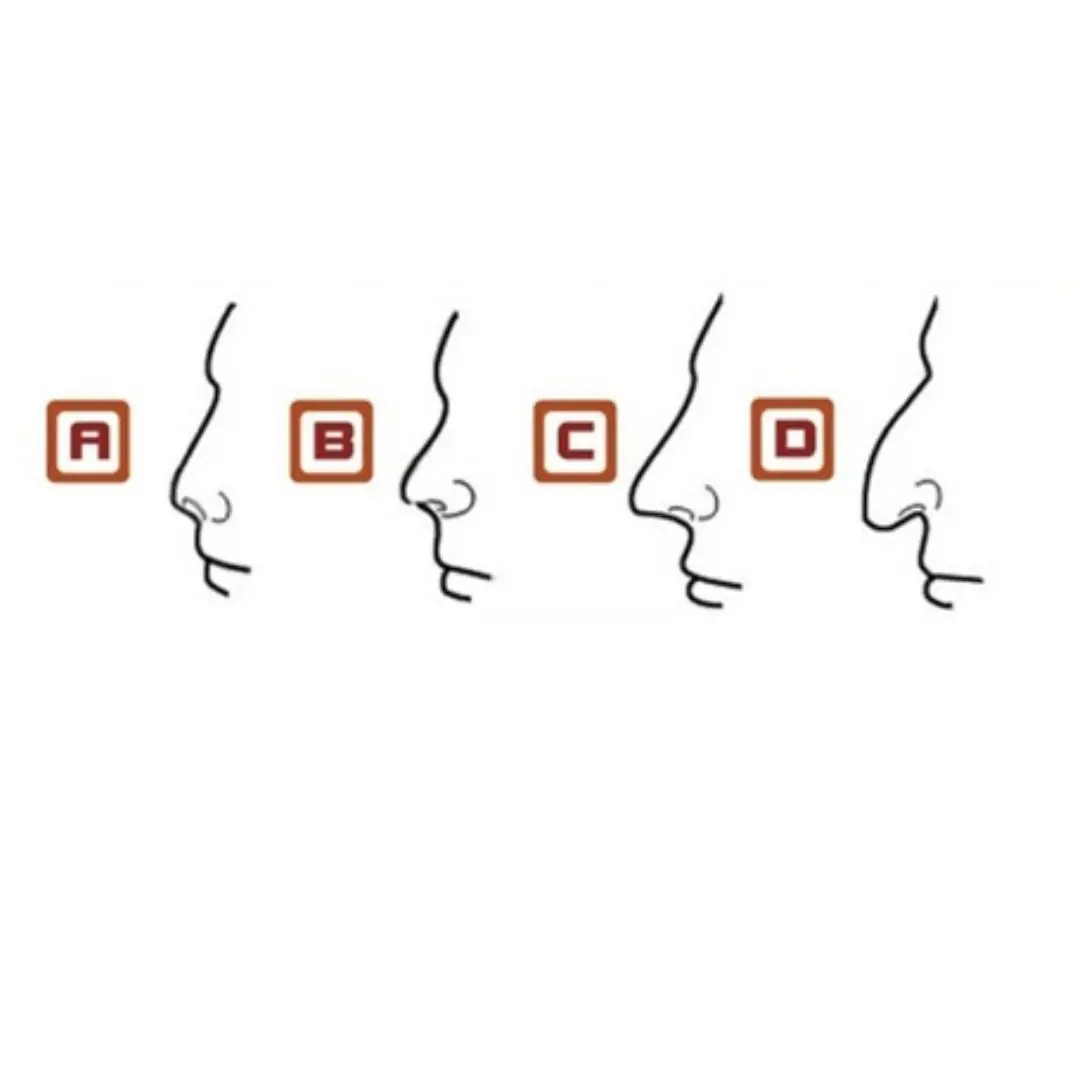
Look at the shape of your nose to predict your destiny and fortune for life

Only people with outstanding thinking can find the answer!

What Is the Spiritual Significance of a Black Cat?
News Post

Loaded Bacon Cheeseburger Tacos: When Comfort Food Gets a Bold Twist
A crave-worthy fusion that combines tacos with classic cheeseburgers.

Olive Oil Production Hub Excavated in Turkey
Researchers say the findings indicate Syedra was not only a regional producer but one of the Mediterranean’s key suppliers in antiquity, challenging earlier assumptions about the city’s economic role.

Why don't Americans eat pork?
We all know that food consumption habits vary greatly among countries.

Why do hotel beds often have 4 pillows?
Typically, hotel beds come with at least four pillows
Here’s What Really Happens When You Sleep with Socks On
Older people may experience issues with blood circulation more often than the younger population.

These 5 groups of people should not eat tofu
Tofu is Nutritious but These 5 Groups Should Avoid It
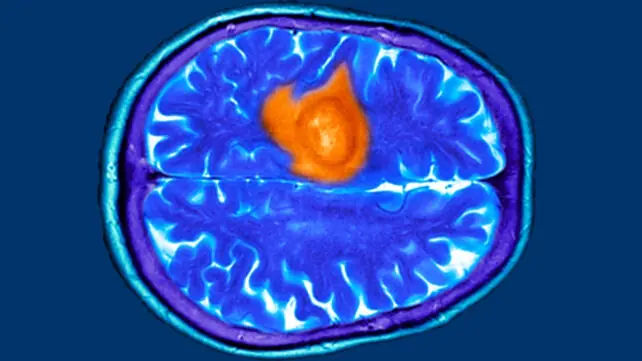
7 Possible Brain Cancer Symptoms Every Woman Should Be Aware Of
7 Possible Brain Cancer Symptoms Every Woman Should Be Aware Of

Plants that should not be planted around the house
Plants that should not be planted because they attract snakes into the house, including very familiar types

Cold-Weather Headaches: A Harmless Chill—or a Silent Warning of High Blood Pressure?
Cold weather often brings complaints of headaches and dizziness, especially in the early morning or late at night.

Is Using Your Phone While Someone Is Talking Considered Rude? The Uncomfortable Truth About Modern Manners
When this habit intrudes into face-to-face conversations, a serious question emerges:

A Word of Advice: Don’t Buy Sweet Potatoes If You Notice These 4 “To.xic” Warning Signs—Eating Them Only Harms Your Body
Sweet potatoes are truly a gift from nature—affordable, filling, and nutritious. But for them to be genuinely good for your health, careful selection is essential.

Three Common Intimate Behaviors in Men That May Raise Cervical Can.cer Risk in Women
Certain intimate habits in men may silently increase women’s cancer risk.

A 50-Year-Old Man Di.es After Eating Leftovers: 5 Foods You Should Never Store Overnight
A tragic case highlights the hidden dangers of improperly stored leftovers.
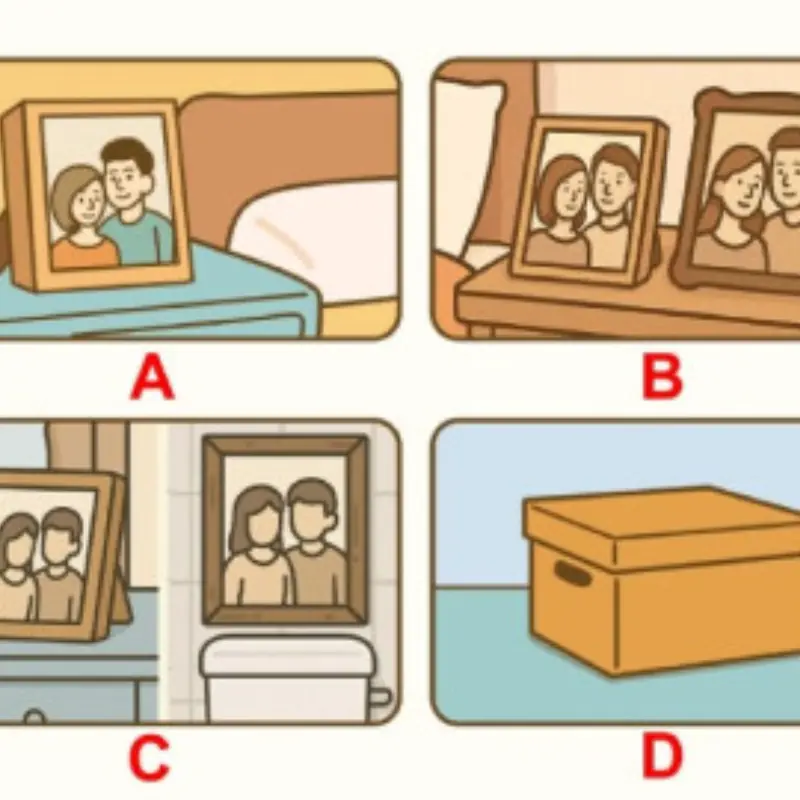
How an Old Photo Can Reveal Your Emotional Intelligence in Love
A simple quiz shows how you balance past feelings and present relationships.
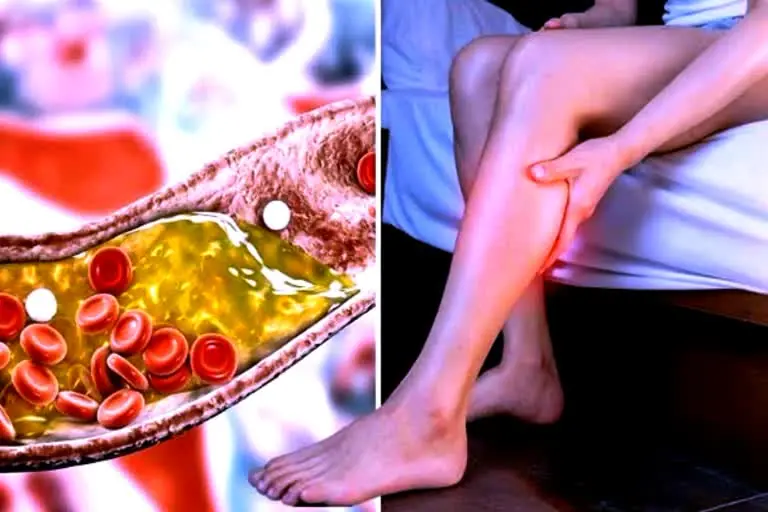
Warning Symptoms of Heart Blockage In Legs And Feet At Night
Warn:ing Symptoms of Heart Blockage In Legs And Feet At Night

Drinking coffee at certain time of day could reduce your risk of de.ath and heart disease

Why Many Couples Choose to Sleep Separately After Turning 50
Separate bedrooms after 50 are more about health than romance.

Will Humans Lose Jobs en Masse Because of AI — or Only the Lazy Will?
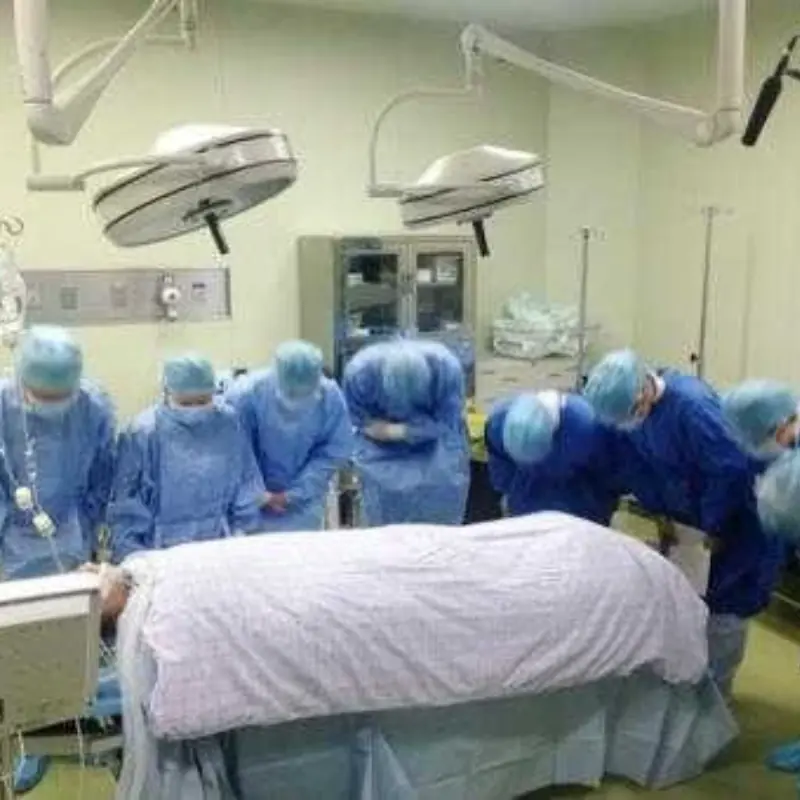
Doctors Warn Parents After 5-Year-Old Dies From Advanced Can.cer
A child’s death prompts doctors to warn parents about unhealthy diets.
Stretch your ring finger with your thumb and hold it for a few seconds. You'll love the reason
Stretch your ring finger with your thumb and hold it for a few seconds. You'll love the reason!