Today’s hottest news: Suzy and Song Hye Kyo publicly “dating”?
On June 22, netizens were abuzz with a series of “dating” photos of Song Hye Kyo and Suzy. The two famous beauties hung out and took pictures of each other. This topic quickly went viral on social media because, until now, Suzy and Song Hye Kyo had never publicly interacted, and netizens were unaware of their friendship.
The shared frame of the two stunning beauties Suzy and Song Hye Kyo has netizens swooning.

The article about Song Hye Kyo and Suzy’s “date” quickly climbed to the second position on the Naver portal. Netizens lavished praise on the visuals and friendship of the two stars: “It’s been a long time since we’ve seen top stars hanging out together,” “Both are exceptionally beautiful and talented, it’s wonderful,” “I didn’t know they were friends”… Many are hoping that Song Hye Kyo and Suzy will co-star in a film soon.
The “date” of Suzy and Song Hye Kyo surprised everyone. The two stars dressed casually, hung out, and took photos of each other. The “Nation’s First Love” looked cute and adorable through Song Hye Kyo’s lens.Song Hye Kyo also looked stunning and graceful through her junior’s lens.
The meeting of two top beauties in Korea became the hottest topic of the day. Suzy looked bright and beautiful, deserving of the title “Nation’s First Love.” Meanwhile, Song Hye Kyo, 13 years older, did not fall short. She stood out with her feminine and graceful beauty.


Since her divorce from Song Joong Ki, Song Hye Kyo has made significant strides in her career. She won the Best Actress award at the 2023 Baeksang Awards for her outstanding performance in The Glory. Additionally, she has expanded her activities into the fashion industry. Song Hye Kyo is currently an ambassador for Fendi and Chaumet and was invited to attend the world’s biggest fashion event, the 2023 Met Gala. She recently completed filming her new movie, The Black Nuns.
Suzy, born in 1994 as Bae Suzy, debuted as the youngest member of Miss A in 2010. She is dubbed the “Nation’s First Love” for her pure and sweet beauty. After the group’s disbandment, she achieved significant success as an actress. Some of Suzy’s notable works include Dream High, Start-Up, Doona, and While You Were Sleeping…
News in the same category


Dispatch Questions Gold Medalist’s Intentions And Honesty Leading Up To Kim Sae Ron’s D.e.ath

Kim Soo Hyun was du.g up for making hidden comments att.ac.king Sulli publicly, taking advantage of her sc.andal to promote his movie

Kim Soo Hyun dated Kim Sae Ron when she was 15 years old, her letter before she pa.s.sed away was rev.ealed

The tr.a.g.e.d.y of Kim Sae Ron

Police Confirm Actress Kim Sae-ron Found De.ad at Home; Investigation Ongoing

Rosé (BLACKPINK) breaks Gangnam Style's record

Will he win the Golden Globe after the Emmy Award?

Actress Zhao Han Ying Zi revealed that her friend is dating Cha Eun Woo

Are Rosé & Evan Mock Dating? The Truth Behind the ‘Toxic Ex’

aespa’s Winter and ENHYPEN’s Jungwon caught in dating rumors

Rosé can't escape from love in 'Toxic Til The End' MV

Actor Park Min Jae passed away suddenly at the age of 32

Looking back at memorable moments of BTS's pet V - Yeontan: 7 years in the blink of an eye

IU and Byun Woo Seok to star in royal romance set in a modern constitutional monarchy

Gong Yoo and Seo Hyun Jin’s new Netflix drama 'The Trunk' sparks heavily divided opinions among viewers
Rosé shares BLACKPINK’s new album plans and her aspirations for more potential solo collaborations

NewJeans terminates contract with ADOR

Lee Dong Hwi and Jung Ho Yeon officially end their relationship after 9 years

Jung Woo Sung revealed to be in a long-term relationship with non-celebrity amid paternity controversy
News Post

If These 4 Foods at Home Start Sprouting, Don’t Throw Them Away: They’re Not To.xic—They’re Even More Nutritious!
Never Smoked but Still Get Lu.ng Can.cer? Doctors Say the Cause Comes from One Thing Almost Everyone Is Exposed To — Especially Asian Women

Can.cer Cells Love These 3 Flavors the Most — Many People Are Shocked to Realize They Eat Them Every Day

3 Vegetables Known as “Natural Li.ver Tonics,” Widely Sold in Local Markets
3 Selfish Habits of Husbands That Increase a Wife’s Risk of Cervical Can.cer: Stop Them Now Before They Harm the Whole Family

Two Fruits That Can.cer Cells “Love”: Read This to Know What to Avoid

Pine Cone Syrup for Beginners: Natural Benefits, How to Make It, and Practical Uses

Doctors Warn: 2 Winter Bathing Mistakes That Increase the Risk of Headache and Stroke

Goodbye fleas, ants, and cockroaches with this home remedy

How Did Song Meiling Live to 106 After a Can.cer Diagnosis at 40? Her Diet May Explain Why
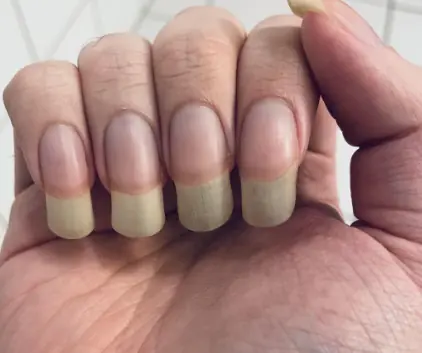
Why do some men grow this nail long?
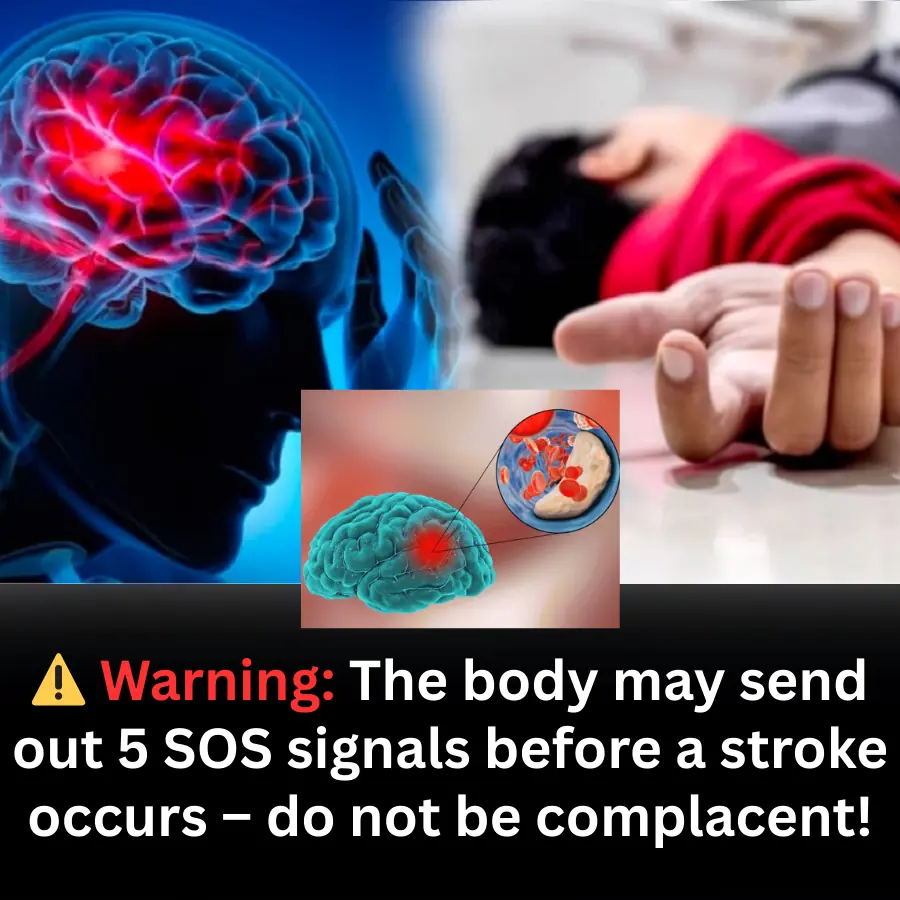
Doctors Warn: 5 Warning Signs the Body May Send Before a Stroke

Female Student Hospitalized With Severe Kidney Infection After a Common Bathroom Habit

Turns out this is what costs us more electricity than anything else

3 Easiest Ways to Get Rid of Mice in Your House

Behind her battle with a rare disease at a young age was a harmful habit many unknowingly practice every day

32 Warning Signs of Magnesium Deficiency You Shouldn’t Ignore
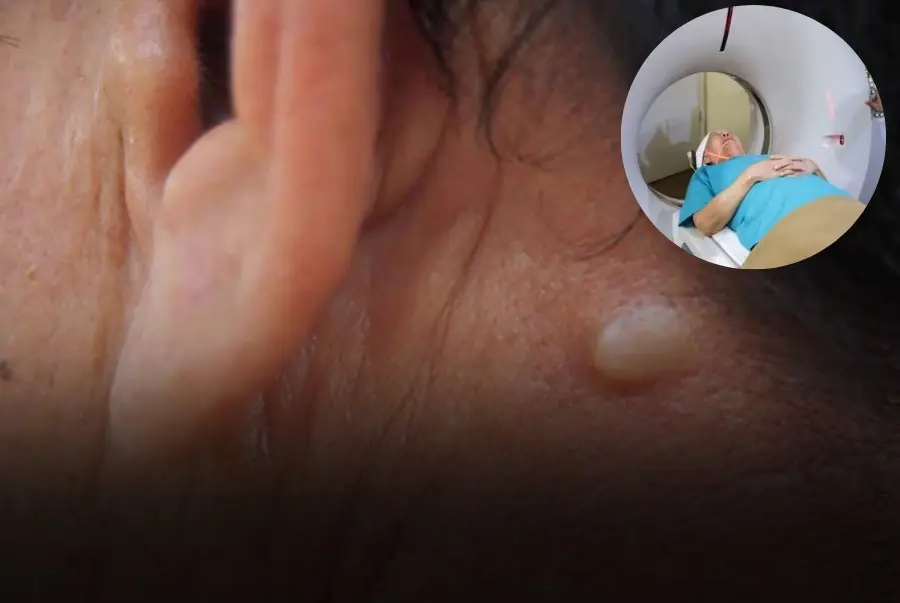
5 Early Signs of Thyroid Can.cer That Are Easy to Recognize

Three Unusual Neck Signs That May Signal a Serious Health Risk