
Despite its nutrition, it must be consumed properly
Health benefits of cabbage
Cabbage is a familiar vegetable, with many different varieties and is sold almost all year round, but the main season is still winter. This vegetable brings many health benefits.
- Good for the heart
Cabbage is a vegetable that contains high levels of potassium, which helps maintain blood circulation and prevent blood vessel blockage. In addition, potassium is also a compound that helps regulate blood pressure.
- Good for the brain
Cabbage provides a lot of vitamin K and anthocynin, which have good effects on the nervous system, helping to increase brain concentration. These nutrients play an important role in preventing nerve damage, helping to prevent age-related memory loss.
- Good for the eyes
The beta-carotene component in cabbage has the effect of improving eye health, preventing macular degeneration and cataracts in the elderly.
- Anti-aging
The vitamin C and sulfur content in cabbage is relatively high, which helps prevent free radicals from damaging cells in the body, helping to prevent aging and prevent diseases.
- Weight loss support
Cabbage contains a lot of water and fiber, which helps create a feeling of fullness for a long time, prevents cravings, and limits snacking. In addition, it also helps prevent glucid in the body from converting into fat, causing weight gain, obesity, and fat accumulation. You can use boiled cabbage (eat both the water and the vegetables) to receive the benefits that this vegetable brings.
Foods that are incompatible with cabbage
- Cucumber
Cucumber and cabbage both have many nutritional ingredients that are beneficial for health. However, when combining these two together, it will reduce the nutritional value of both, affecting the body's absorption of vitamin C.
- Animal liver
Cooking cabbage with animal liver will reduce the nutritional value of both foods.
- Apple
Eating purple cabbage with apples also affects the body's ability to absorb nutrients.
- Mangosteen
Eating mangosteen and cabbage at the same time will increase the burden on the digestive system, hindering the body's absorption of nutrients.
People who should not eat cabbage
- People with goiter, hyperthyroidism
Cabbage is a vegetable with a relatively high content of the antioxidant glucosinolate. Under certain conditions, this substance will be broken down into isothiocyanate and thicyanate, which can cause thyroid disease.
In addition, cabbage also contains goitrin. This substance has antioxidant effects but can also cause goiter.
News in the same category


Flight Attendant Reveals the Real Reason Cabin Crew Sit on Their Hands During Takeoff

Should You Wash Meat and Fish Bought from the Supermarket?

If You Don’t Unplug These 5 Electrical Devices at Home, Your Electricity Bill Could Skyrocket!

At the market, if you see these 4 types of vegetables, don’t hesitate—buy them immediately

Flip the Bottle: The Simple Trick to Tell Real Honey From Fake—Experts Warn Shoppers to Stop Buying Blindly

Why You Should Never Place the Head of Your Bed Toward These Two Directions: Feng Shui Experts Call It a Major Prosperity Taboo

Shower First or Wash Hair First? Doctors Warn: The Wrong Order May Trigger Dangerous Health Risks

Surprising Nutrition Insight: 4 Sprouted Foods That Become Even Healthier — Not Toxic

Doctors Warn: Going to Bed Too Early May Be Harmful for Seniors — People Over 65 Should Sleep at This Recommended Time

Experts Warn: 4 Fruits Most Commonly Sprayed With Pesticides — Shoppers Should Avoid Buying Them Hastily
The finger you cut first might say more about you than you think

If Your Parent Shows These 3 Signs, They May Be Nearing the End of Life. Prepare Yourself for What’s to Come

When a Wife’s Intuition Speaks: Signs Your Husband May Be Having an Affair
The Story of Two Exhausted Surgeons After a 32-Hour Operation: A Symbol of Sacrifice in Medicine

When Someone Close to You Passes Away, Never Throw Out These 4 Important Items

Ancient Wisdom Explains: “Don’t Buy Pork Neck, Don’t Buy Crucian Carp”—The Real Reasons Revealed Today

A Simple Method Keeps Rice Fresh for One Year Without Worms or Weevils — Many People Regret Not Learning It Sooner

Charging Your Phone to 100% and Unplugging It Is a Common Mistake: Experts Share the Correct Method to Double Battery Lifespan

The “Miracle Herb” Chinese Locals Praise: Abundant on Roadsides but Often Feared and Overlooked
News Post
Sheet Pan Gnocchi with Zucchini, Tomatoes, and Bell Peppers

Mediterranean Chickpea Salad with Feta

3 types of shirts you should never wear to a funeral

5 groups of people should limit shrimp intake
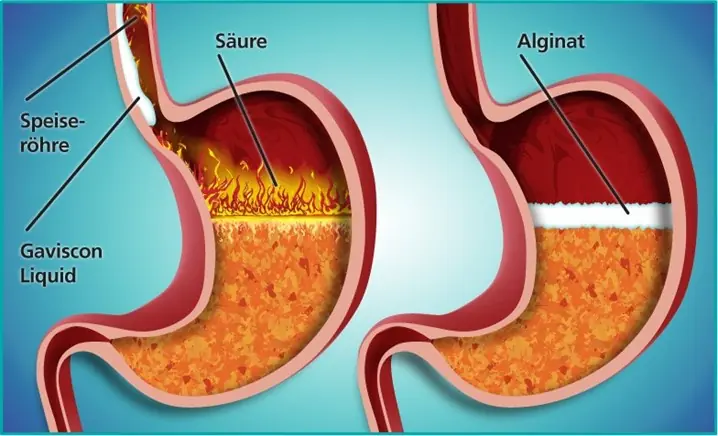
8 Red Flags That Your Stomach Problem May Be Advanced — See a Doctor Early

Creamy Crab and Shrimp Seafood Bisque
These 10 Symptoms Could Mean Your Kidneys Are Failing—Act Now

What is the safest time to bathe to protect your health and reduce str.oke risk?

Flight Attendant Reveals the Real Reason Cabin Crew Sit on Their Hands During Takeoff

Drooling during sleep: A small sign that may point to bigger health issues

4 anti-aging dietary principles you should apply

Roasted Beetroot and Avocado Salad with Feta
Pan.creatic can.cer: 10 early warning signs you should never ignore

Avocado Berry Salad with Nuts & Greens

Doctors Reveal the Truth About Avocado That Most People Don’t Know

Mediterranean Meatball Bowl with Roasted Potatoes & Tzatziki

Churro Caramel Crunch Cupcakes

Creamy Chicken Vegetable Soup
His whole body was itchy, he thought it was an allergy. But he was diagnosed with
