Mistakes to avoid when using a water purifier
The "misunderstandings" that turn water purifiers into breeding grounds for illness—stop them now to protect your family.
Main Causes of Water Filter Malfunctions
Using a Filter for Too Long
Over time, the filter loses its ability to kill bacteria, rendering the water purifier ineffective.
Not Installing the Water Outlet in the Hot Water Pipe
The water outlet should never be installed in the hot water pipe. This can cause serious damage, including pipe leakage and rapid deterioration.
Not Replacing the Filter
Replacing the filter regularly ensures safe, mineral-rich water. If not replaced, the filter becomes ineffective, and the water purifier becomes dangerous. Always seek professional help to fix issues and ensure proper installation.
Boiling Filtered Water Before Drinking
It’s a common misconception that boiled water is always safer. In fact, water from a good purifier already meets health standards and can be consumed directly without boiling. Boiling it can reduce beneficial minerals, making the water less healthy.
Choosing Water Purifiers with Too Many Filters
More filters do not necessarily mean better filtration. A good purifier typically has a maximum of five filters. Adding more filters may remove beneficial minerals. Choose filters that are specifically designed for your water needs, like mineral-enriching filters.
News in the same category


Why Placing Ginger by Your Bedside Can Benefit Your Health

Can Lemon Seeds Really Save a Snakebite Victim in One Minute? Doctors Warn Against a Dangerous Myth

5 Common Refrigerator Mistakes That Can Multiply Bacteria by 10 Times
A Common Mistake Countless Families Don’t Even Know They’re Making

In winter, drying thick clothes is a slow process—and sometimes, they end up smelling bad.

The real electricity th.ief in your home

Scientists Finally Reveal a Sho:cking Answer to the ’Chicken-or-Egg’ Dilemma

Two-Way Mirrors: The Privacy Risks Most People Don’t See

Put ginger next to your pillow when sleeping: A simple secret for good health and sleep
Why do dogs ba.rk and bi.te some people but not others? There's always a reason!

Packed with powerful nutrients, these 3 humble vegetables are hailed in Japan
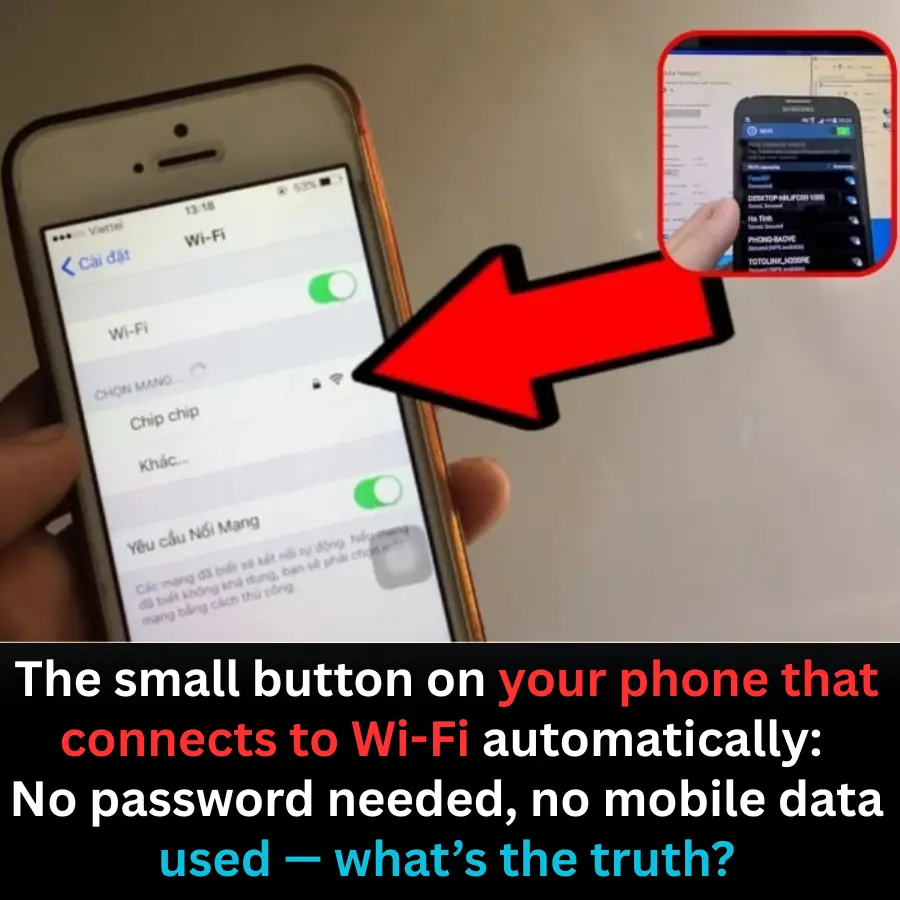
The small button on your phone that connects to Wi-Fi automatically: No password needed, no mobile data used — what’s the truth?

Applying Medicated Oil to the Soles Before Sleep: A Traditional Practice with Potential Benefits

When a lizard visits your house that’s a sign...

Even if you remain single forever, never marry a man from these 4 types of families

You Sleep on It Every Night — But Could It Be Exposing You to Toxins?

Out at Night and See This Scene? Don’t Get Closer

The Anti-Can:cer Vegetable Ranked Best in the World by U.S. Experts

3 vegetables that may cause can.cer avoid them now
News Post

Sweet potato lovers should read this article - it will change your life Knowing this now is not too late!

How to Tell Real Baby Formula from Fake: What Every Parent Needs to Know

The Tiny Seat Belt Button You’ve Probably Never Noticed — Here’s What It’s For

The Hidden Power of Orange Peels: A Forgotten Treasure You Shouldn’t Throw Away

The Food That Rejuvenates Skin from the Inside: One Bowl Is Better Than 10 Face Masks

5 early can.cer symptoms experts say are commonly ignored

Croissant Breakfast Plate with Scrambled Eggs & Avocado

Why Horse Chestnut Is So Effective for Swollen Legs
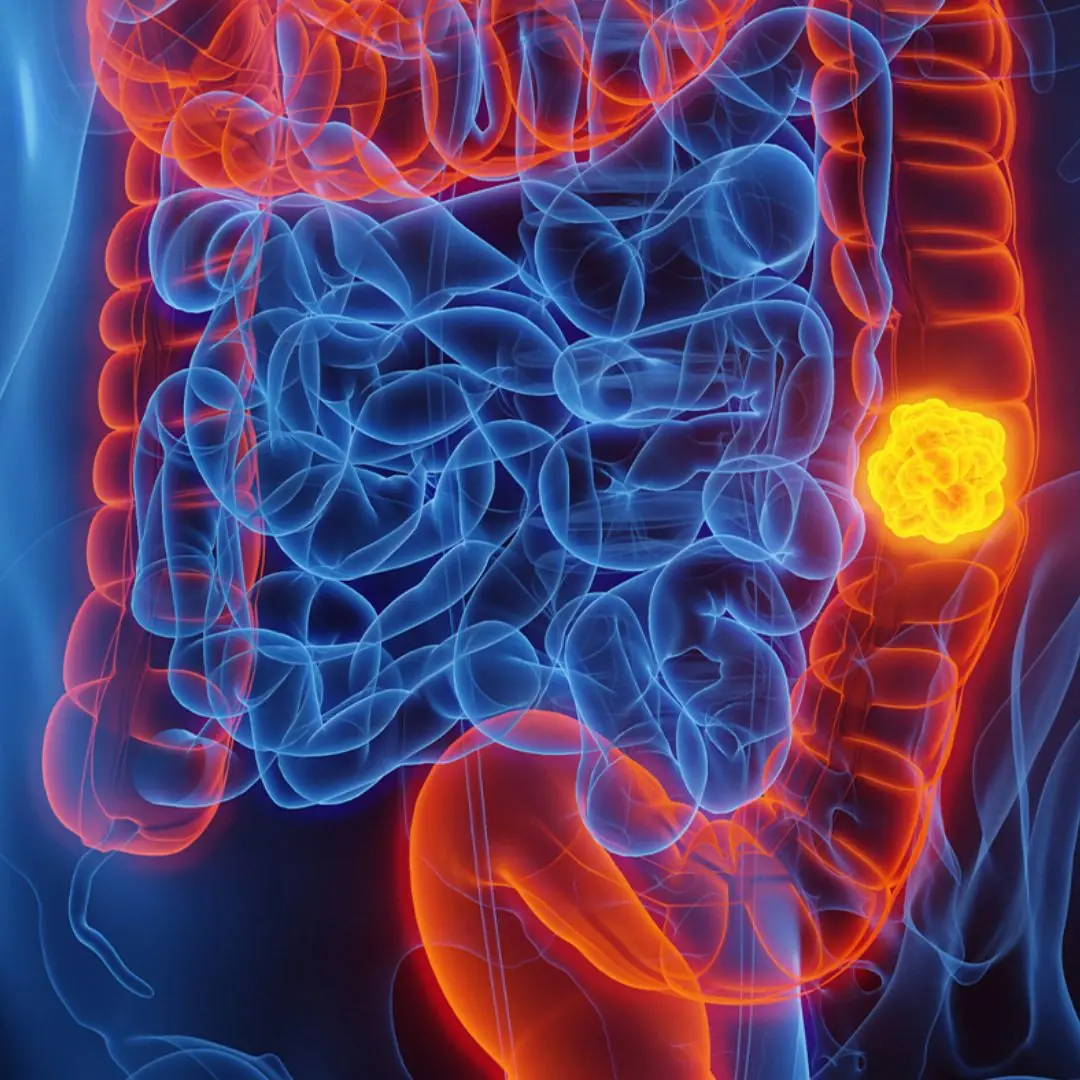
She Thought It Was Nothing: Woman with Stage - Four Colon Cancer Reveals 5 Symptoms She Overlooked

Refreshing Peach Lemonade
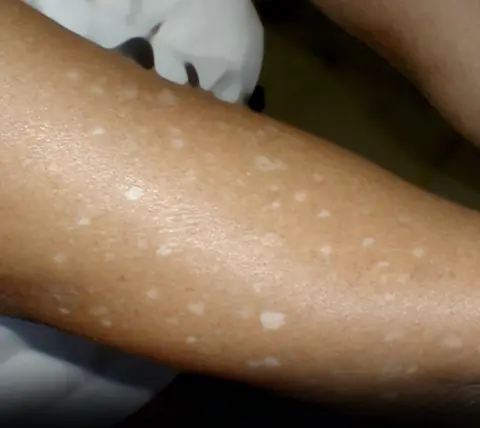
Have you noticed small white spots on your arms or legs… and you don't know what they are?

Doctors reveal that eating guava causes...
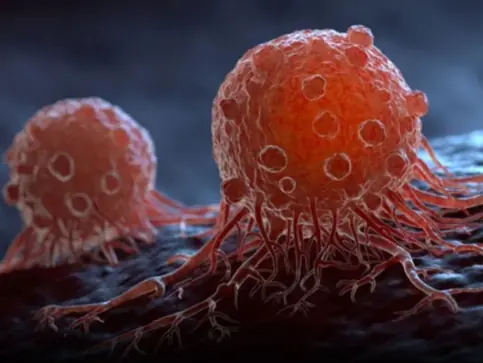
Discover 20 signs of cancer that most women ignore
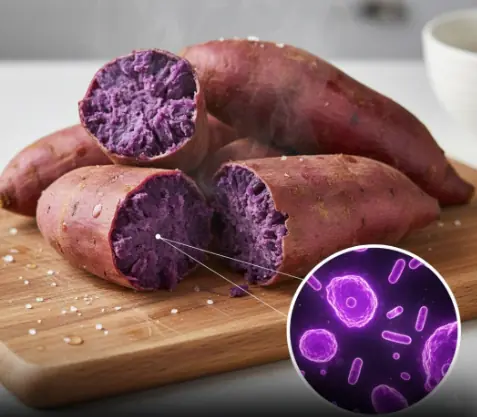
Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells
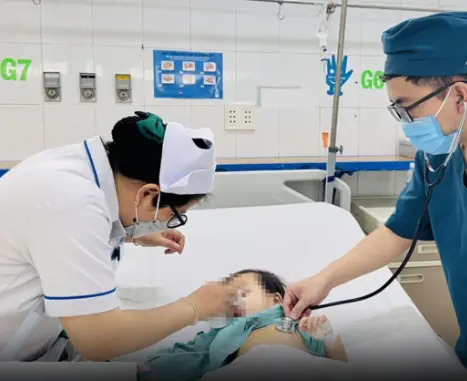
Parents’ Common Feeding Habit Leads to Stomach Cancer in 2-Year-Old Boy
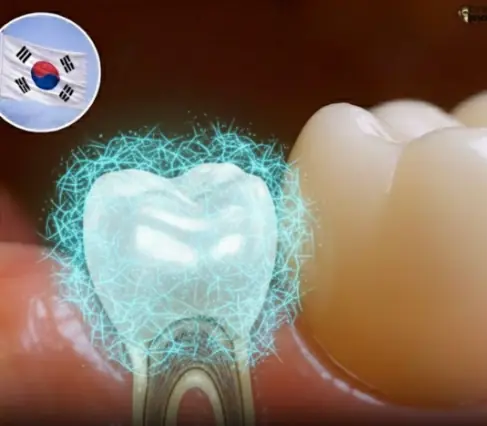
Revolutionary Microneedle Patch Offers Hope for Natural Tooth Regrowth
5 Early Symptoms of Stomach Cancer That Help with Early Detection

Night Leg Cramps? The Real Reason Might Surprise You
Advanced-Stage Can.cer May Show 4 Unusual Warning Signs — Seek Medical Care as Early as Possible